Defense of Animal Agriculture
NOTE: The Blue Ribbon Study Panel on Biodefense is now the BIPARTISAN COMMISSION ON BIODEFENSE

The Threat To Food And Agriculture
The Threat Of Zoonoses
U.S. Agrodefense Today
Federal Structural Organization
Artificial Policy Divisions Hamper Progress
Recognition Of The Threat By High-Level Leadership
Law Enforcement And Attribution Of Attacks
Commissioners
Joseph I. Lieberman, Chair
Thomas J. Ridge, Chair
Donna E. Shalala
Thomas A. Daschle
James C. Greenwood
Kenneth L. Wainstein
Ex Officios
Yonah Alexander, PhD
William B. Karesh, DVM
Rachel Levinson, MA
I. Lewis Libby, JD
Gerald W. Parker, DVM, PhD
George Poste, DVM, PhD, DSc
Tevi Troy, PhD
Commission Staff
Ellen P. Carlin, DVM, Co-Director
Asha M. George, DrPH, Co-Director
Robert H. Bradley, Policy Associate
Patricia Prasada-Rao, MPH, Panel Coordinator
Patricia de la Sota, Meeting Coordinator
Katherine P. Royce, EcoHealth Alliance Intern
Acknowledgements
The Commission thanks Hudson Institute for serving as our fiscal sponsor. The Commission also thanks Kansas State University for hosting our special focus meeting on agrodefense, and the many experts that spoke at this meeting, some of whom came from great distances to do so. We are grateful to the many individuals both inside and outside of the federal government who provided input into the report throughout its development. For peer review of complete late-stage drafts, we would like to thank Dr. John Goldberg of Science Based Strategies; Dr. C. J. Mann of Empryse Group; Dr. Monique K. Mansoura of the Massachusetts Institute of Technology; Dr. Tracey McNamara of Western University; and other anonymous reviewers. Their responses were exceptionally beneficial but should not be presumed to constitute an endorsement. The Commission’s Ex Officio members also provided substantial technical input. Finally, the Commission gratefully acknowledges the financial support provided by its donors, without which this report would not be possible.
Executive Summary
The increasing rate of emerging and reemerging zoonotic disease, along with threats and attempts by those with nefarious intent to attack food and agriculture, point to the need to exert more effort to eliminate vulnerabilities and reduce consequences associated with America’s agricultural sector. The Food and Agriculture (F&A) critical infrastructure sector produces, processes, and delivers the systems and commodities that feed billions of people and animals throughout the United States and globally. In 2015, the agriculture, food, and related industries contributed $992 billion (5.5%) to U.S. gross domestic product (GDP), making it one of the largest sectors of the U.S. economy. Given its critical importance to food safety and availability in the United States and around the world, protecting this sector is a matter of national security. Federal agencies; state, local, tribal, and territorial (SLTT) governments; academic institutions; and industry partners all contribute to and are responsible for this vast enterprise. Our lives, culture, economy, and livelihood depend on their efforts.
 In its 2015 A National Blueprint for Biodefense: Leadership and Major Reform Needed to Optimize Efforts, the Bipartisan Commission on Biodefense (formerly the Blue Ribbon Study Panel on Biodefense) determined that national biodefense lacked centralized leadership, interagency coordination and accountability, collaboration with non-federal stakeholders, and incentives for innovation sufficient to achieve needed capabilities and maximize mission effectiveness. With its series of special focus reports, the Commission undertakes in-depth examinations of particular biodefense topics of concern, considers how the recommendations it made in the Blueprint for Biodefense apply to these topics, and adds detail and new action items in keeping with its existing recommendations. This special focus report is the first in the series, and reflects the Commission’s evaluation of threats to animal agriculture, a critical infrastructure component central to the health and well-being of the population and the security of a major element of the national economy.
In its 2015 A National Blueprint for Biodefense: Leadership and Major Reform Needed to Optimize Efforts, the Bipartisan Commission on Biodefense (formerly the Blue Ribbon Study Panel on Biodefense) determined that national biodefense lacked centralized leadership, interagency coordination and accountability, collaboration with non-federal stakeholders, and incentives for innovation sufficient to achieve needed capabilities and maximize mission effectiveness. With its series of special focus reports, the Commission undertakes in-depth examinations of particular biodefense topics of concern, considers how the recommendations it made in the Blueprint for Biodefense apply to these topics, and adds detail and new action items in keeping with its existing recommendations. This special focus report is the first in the series, and reflects the Commission’s evaluation of threats to animal agriculture, a critical infrastructure component central to the health and well-being of the population and the security of a major element of the national economy.
The Commission views protection of agriculture – the cultivation and breeding of animals and plants for food, fiber, and other products used to sustain human life – as a critical part of the overall biodefense mission space. While nearly all the Commission’s Blueprint for Biodefense recommendations apply to agrodefense, some are especially important for the mission and deserve particular attention at this time. The goal of this report is to elucidate a few key, persistent challenges and to propose solutions. This report does not address every challenge in agrodefense. It emphasizes that intersection of issues which reflect the underlying principles of the Blueprint for Biodefense, and which have been inadequately evaluated or discussed in other fora. This report does not directly assess threats to food (including food safety issues) or to plant agriculture, two areas of great import that rightfully deserve their own substantive analyses. Neither does it address food security (access to food), another important topic. These topics were beyond the scope defined for this special focus report. Additional areas for oversight consideration are included at the end as proposed congressional hearings.
The findings and recommendations herein are structured along the same thematic lines as the Blueprint for Biodefense: Leadership, Coordination, Collaboration, and Innovation. Recommended actions are listed in the Summary of Proposals for the Executive Branch and the Summary of Proposals for Congress, and are designed to align directly to recommendations in the Blueprint for Biodefense.
Leadership
As assessed in our previous report, White House-level political leadership is necessary to elevate biodefense as a critical national and federal imperative. As recommended, the Vice President, in conjunction with strong congressional champions, could better drive priorities and activity across the large, unwieldy enterprise of agricultural defense.
Agricultural defense is a broad and complex mission space that necessitates the significant involvement of most federal departments and agencies. Presidential Policy Directive 21 places the Department of Agriculture (USDA) and the Department of Health and Human Services (HHS) as the federal leads for the F&A critical infrastructure sector. Roles and responsibilities under the U.S. Code and other authorities are not necessarily coordinated, however, nor are authorities necessarily exercised in a way that has prioritized needed activity.
The ultimate ownership of F&A by the private sector, and its significant contribution to SLTT and international economies, necessitates substantial federal collaboration with non-federal stakeholders. White House-level leadership is critical to minimize overlap, identify mission gaps, and coordinate effort. The White House should ensure that the National Biodefense Strategy addresses threats to food and agriculture. The President and Congress should ensure that detailed agrodefense expenditures are incorporated into a cross-cutting biodefense budget analysis.
Coordination
Agricultural outbreaks may result from natural events or from deliberate actions. Coordination between animal health (a USDA mission), and law enforcement (a Federal Bureau of Investigation, or FBI, responsibility), is critical. Sharing information among these and other interagency entities as well as non-federal stakeholders is necessary to focus attention on the most relevant threats and ensure that prevention and response measures are aligned with those threats.
The Commission recommends increased coordination between the USDA and FBI. Further, since the FBI deems all domestic incidents of foreign animal diseases suspicious, law enforcement and health officials should conduct joint investigations of all such outbreaks. The development of an updated Food and Agriculture Incident Annex (FAIA) will be a critical step toward improving preparedness for agricultural outbreaks. Any revision must prioritize planning for both natural and intentional events.
The Federal Emergency Management Agency, the USDA Animal and Plant Health Inspection Service, and the FBI should ensure that any update to the FAIA recognizes and addresses the investigative mission of the FBI, and clearly directs other federal departments and agencies to support inquiries into suspected acts of agricultural crime and terrorism.
Collaboration
Effective overall homeland security depends on successful collaboration among federal and non-federal stakeholders. The same is true for agrodefense, especially regarding early detection and surveillance efforts to characterize and prevent further spread of disease. The early detection of infectious disease outbreaks is one of the most important means we have for mitigating their impacts and shortening the duration of response. This detection should occur at the level of livestock production, but also in wildlife.
Although the nation has made great strides, it still falls critically short in rapid biodetection, diagnosis, and integrated biosurveillance of outbreaks. Biodetection is hampered by an insufficient focus on rapid pen-side diagnostics, and insufficient investment to develop new wildlife disease detection technologies and validate existing tests. Although improving, biosurveillance remains perpetually challenged by information sharing problems. Much of the data are owned by the private sector, thus requiring protected information policies that incentivize sharing. Success also depends on the cooperation of federal and state agencies. White House leadership could provide the basis for the coordination and collaboration necessary to optimize the needed functions of biosurveillance collection, integration, and analysis. The White House should consider the full scope of wildlife surveillance activity that would benefit wildlife, livestock, and human health, and direct relevant departments to develop a commensurate budget request. The National Security Council should direct interagency partners to develop a standard of quality by which the value of investment in biosurveillance can be measured. Congress should fund and facilitate enhanced opportunities for data collection from livestock and wildlife, including through increased appropriations to the USDA National Wildlife Disease Program.
Innovation
Ultimately, the current paradigm for disease response is insufficient to protect the sector. The nation needs new ideas and scientific solutions to drive agrodefense approaches beyond their current limitations. One example would be to increase funding to the National Veterinary Stockpile to demonstrate a market commitment to procurement the way the BioShield Special Reserve Fund was designed to do for human medical countermeasures.
To meet the requirements of Homeland Security Presidential Directive 9, far greater investment in advanced research and development is also necessary. The nation requires focused investment in pen-side, innovative diagnostic technology, and in better laboratory-based technology to enable rapid assessment for SLTT animal health officials, enabling earlier decision-making. The USDA should further develop its vaccine use policy for avian influenza and other high-consequence diseases, basing these policies on the use of platform technologies for rapid diagnostics and vaccines in response to outbreaks.
Additionally, DHS and USDA should develop a business plan for the operation of the National Bio- and Agrodefense Facility. This plan should engage the public and private sectors; consider domestic and global markets for agrodefense research and development; and identify a dollar figure that defines both need and opportunity.
The President’s Fiscal Year 2018 budget request would eliminate all agriculture and animal-specific research by the DHS Science and Technology Directorate. This signals a substantive diminishment of support from the Executive Branch for agriculture and agrodefense research.
The Administration must improve agrodefense efforts to prevent or combat a major agro-disease outbreak. Although accounting for only 5% of GDP, food safety and food access affects 100% of the population. F&A are increasingly vulnerable to large-scale disease outbreaks that could significantly impact the economy, and which could also threaten the security of the population. The Panel believes that current government efforts should be assessed and redirected as outlined in this report per the forthcoming National Biodefense Strategy. Federal investment in agrodefense must focus on prevention and early identification to reduce or prevent the incursion of major costs and losses.
Like homeland security in general and biodefense in particular, the interagency nature of agrodefense means that many congressional committees oversee agrodefense efforts. These committees should both continue and expand previous efforts and increase their direction to the Executive Branch. The Farm Bill provides a significant opportunity every five years to accomplish this legislatively.
Leadership
- Ensure that the National Biodefense Strategy and its implementation plan address threats to food and agriculture, including any gaps in Homeland Security Presidential Directive 9 implementation;
- Collect detailed agrodefense expenditures and provide them to Congress as part of an annual biodefense data call;
Coordination
- Formalize cooperation between the federal agriculture and law enforcement sectors to ensure that outbreaks are evenly addressed by both, in particular through the next iteration of the Food and Agriculture Incident Annex (FAIA);
- Ensure that the FAIA describes the critical role played by the nation’s fusion centers, and is regularly exercised at the state level;
- Develop a standard of quality for biosurveillance;
Collaboration
- Determine the optimal scope of wildlife disease surveillance activity and enhance support for the National Wildlife Disease Program commensurate with that need;
- Enhance collaboration among federal, state, local, tribal, territorial, and private sector entities that collect animal health data;
- Finalize the rule for the National List of Reportable Animal Diseases and incentivize rigorous reporting;
Innovation
- Assess the ability of the National Veterinary Stockpile to meet the mandates of Homeland Security Presidential Directive 9, request budgets commensurate with the threat, and invest in countermeasure development, procurement, and usage policy based on the identified need;
- Devote sufficient resources to diagnostics, including rapid diagnostics, for the National Veterinary Stockpile;
- Establish an antigen bank for foot-and-mouth disease virus; and
- Develop a business plan for the National Bio- and Agrodefense Facility that prioritizes public-private partnerships.
Leadership
- Require the identification of agrodefense expenditures across the federal government;
Collaboration
- Commit to a more realistic funding plan for federal wildlife surveillance efforts, and facilitate increased data collection from livestock and wildlife populations;
- Assess the authorities of the Department of Homeland Security and the Department of Agriculture to further collaboration with other public and private stakeholders that collect animal health data, and take necessary steps to support those efforts;
- Continue funding the National Animal Health Laboratory Network at no less than current authorized levels, with the possibility of additional funds should they be needed to fulfill the Network’s mission;
Innovation
- Establish a prevention fund for animal health disease and disaster programs; and
- Authorize the National Veterinary Stockpile, and require annual progress assessments toward requirements.
Introduction
The Threat To Food And Agriculture
The Food and Agriculture (F&A) critical infrastructure sector produces, processes, and delivers the systems and commodities that feed billions of people and animals throughout the United States and overseas.1 In 2015, agriculture, food, and related industries contributed $992 billion (5.5%) to the U.S. gross domestic product (GDP).2 As one of the largest sectors of the U.S. economy, protecting this infrastructure is a matter of national security.
Agriculture, the cultivation and breeding of animals and plants for food, fiber, and other products, is central to American culture, economy, wellbeing, and livelihood. Because of its importance, agriculture is a target for terrorism, warfare, and criminal activity.3,4 The geographically dispersed yet industrially-concentrated nature of the sector makes it an especially vulnerable target. Farms dot the landscape in every state; livestock are often concentrated in specific locations; and lethal and contagious biological agents that impact plants and animals are more numerous even than those that directly impact human beings.5
As with other critical infrastructure sectors, criminals, terrorists, and enemy combatants may target F&A because disruption of this sector can lead to significant negative effects on the populations it serves. Al Qaeda has stated on numerous occasions that it seeks to impact the economies of those it considers to be its enemies, including with agricultural attacks. Targeted destruction of F&A critical infrastructure is a standard, long-standing, and effective element of warfare, with records of chemical and pathogenic attacks dating back to World War I.6 An outbreak in 2011 of a rare strain of E. coli O104:H4, first identified in northern Germany, spread to 16 countries including the United States, resulting in 4,321 cases of illness and 53 deaths.7 Although initially assumed to have a natural origin, epidemiological evaluation later concluded that an accidental or intentional introduction of contaminant into fenugreek seeds was plausibly responsible.8 The use of biological weapons to attack agriculture could result in billions of dollars in losses. Naturally occurring outbreaks in the United Kingdom of foot-and-mouth disease (FMD) in 2001 and bovine spongiform encephalopathy (BSE) in 1996-7 cost the United Kingdom £8.6 billion (about $14 billion)9 and £2.5 billion (about $3.2 billion), respectively.10 Bioterrorism could easily do the same.
Criminals also target the F&A sector. Documented criminal activity has included theft of expensive foods, hybrid seeds, and hay; growth of poppies for opium; murder of farmers; rustling of cattle and other animals (e.g., bees); burglary of valuable metals; and stealing fertilizer elements (e.g., anhydrous ammonia, ammonium nitrate) that can be used to produce methamphetamines and explosives.11
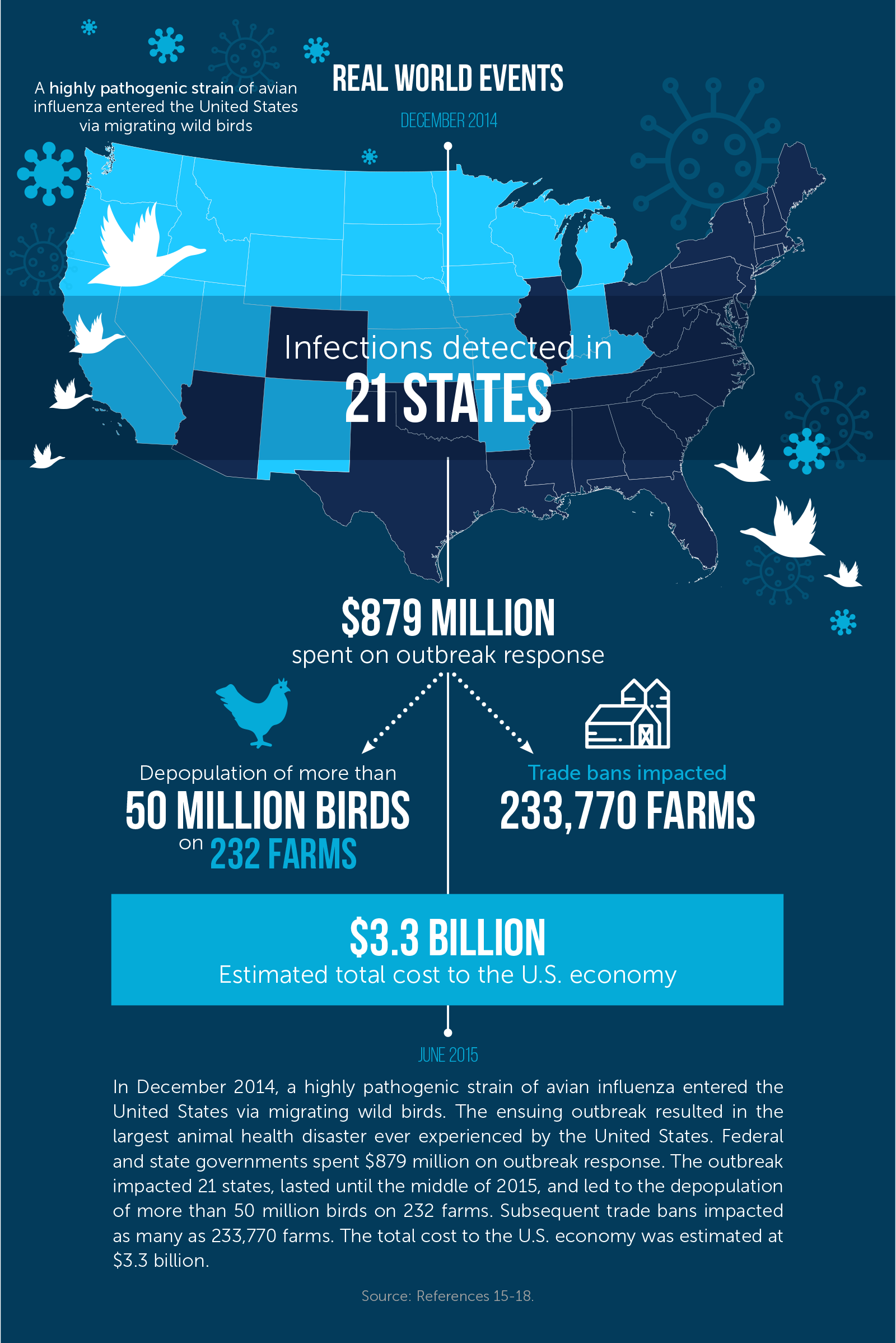
Naturally occurring disease outbreaks remain a persistent challenge. Outbreaks of highly pathogenic avian influenza (HPAI) have led to the deaths of more than 67 million birds in the United States since 1983.12 In December 2014, a highly pathogenic strain of avian influenza entered the United States via migrating wild birds. (Wild birds play a key role in spreading these influenza viruses, such as when they move from northeast Asia into the west coast of North America on their long-distance migration routes.13) The ensuing outbreak resulted in the largest animal health disaster ever experienced by the United States.14 The outbreak lasted until the middle of 2015, ultimately affected 21 states, and led to the depopulation of more than 50 million birds on 232 farms.15 Subsequent trade bans impacted as many as 233,770 farms.16 The total cost to the U.S. economy was estimated at $3.3 billion, with the turkey sector losing $1.1 billion and the egg sector $2.2 billion.17 Federal and state governments spent $879 million on outbreak response.18
HPAI strains can also place humans at significant risk if the strains develop the capacity to spread from poultry to people. The public health community is concerned about possible mutations that would allow these viruses to spread in this fashion. Each case of animal infection during a large-scale outbreak is another opportunity for such a mutation to occur. Further, all avian influenzas can threaten egg production, thereby endangering the supply of human influenza vaccine and other vaccines that depend predominantly upon egg-based culture methods.
The genetic code of the 2009 H1N1 influenza pandemic arose in part from other influenza strains circulating in wild birds and commercial pigs. Media use of the misnomer “swine flu” created misplaced concern among the public over food safety. While human health was never at risk from pork consumption, the pork industry was negatively impacted: consumption declined, sales dropped, hog prices fell, futures prices on the Chicago Mercantile Exchange plunged, and several countries banned U.S. pork imports.19 Inaccurate media linkage of H1N1 to swine cost the U.S. pork industry $200 million.20
Porcine epidemic diarrhea virus (PEDv) and porcine deltacoronavirus (PDCV) emerged for the first time in the U.S. domestic swine population with lethality and ferocity in 2013 and 2014. These swine enteric coronavirus diseases (SECD) cause acute and rapidly spreading diarrhea that does not affect humans, but which can result in 50-80% mortality in piglets.21 PEDv, in particular, results in diarrhea, vomiting, and high morbidity in a herd, and high mortality (90-95%) in piglets. In 2013, PEDv cost the U.S. pork industry returns of $481 to $929 million.22 Although U.S. Department of Agriculture (USDA) guidelines should have been sufficient to control these outbreaks, the USDA did not take regulatory action against SECD immediately. As a result of this, the USDA cannot conclusively determine where or how either virus entered the United States.23 The Federal Bureau of Investigation (FBI) was not contacted to conduct an evaluation of the potential for an intentional (criminal or terrorist) origin for the outbreak.
The Threat of Zoonoses
Among the biological threats for which the U.S. Department of Homeland Security (DHS) has issued a Material Threat Determination, all but one (smallpox) are zoonotic, meaning the disease can move between animals and people. Many major infectious disease outbreaks over the last 10 years (e.g., Ebola, Severe Acute Respiratory Syndrome (SARS), Middle East Respiratory Syndrome (MERS)) have originated in animals. Three-quarters of emerging infectious diseases are, in fact, zoonotic in nature. While most of these originate in wildlife, livestock can also act as conduits for infection. The recent U.S. avian influenza outbreaks did not affect humans, but other avian influenza strains in Asia have infected thousands of people; the H7N9 strain alone has infected more than 1,300 people since 2013.25
While influenza is the most likely virus to cause a pandemic, myriad other viruses cross over from wild animals into human populations. These viruses will continue to create pandemics. In 2003, the emergence of a previously unknown and virulent coronavirus, termed SARS, caused a rapid outbreak in Asia. It is believed to have jumped from bats to an intermediate animal and then to people. SARS quickly incapacitated tourism and trade as the outbreak spread as far as Canada. The economies of China, Hong Kong, Singapore, and Taiwan lost approximately $13 billion in GDP collectively, despite the relative paucity of cases (7,000) and fatalities (700).26 Other global economic costs were as high as $40 billion.27 The cost of patient treatment is not the predominant element in these estimates; the actual costs of SARS were the economic shocks resulting from shifts in human behavior. Ultimately, the infection spread to 29 countries.28 Authorities were finally able to contain its spread, but the rapidity with which the virus breached hemispheres revealed the extreme interconnectedness of human health in the modern era. The more recent Ebola and Zika outbreaks reinforce this fact. According to Dr. Ali Khan, former director of the Office of Public Health Preparedness and Response at the Centers for Disease Control and Prevention (CDC), the primary threat to the health security of this nation remains a zoonotic disease.29
U.S. Agrodefense Today
In 2004, Dr. Roger Breeze, former director of the USDA biosafety level 3 laboratory at the Plum Island Animal Disease Center (PIADC) wrote:
Our national policy for inadvertent and deliberate foreign animal disease introductions should be simple: we will minimize direct and indirect economic impacts, and we will not engage in mass slaughter. Fortunately, most of the tools and technologies to permit such a policy already exist. We now have rapid, on-farm tests for these diseases; effective vaccination strategies; Internet-based command, control, and communication systems; and the means to track animal products from farm to table, even internationally… If we choose this way forward, there will be little point in deliberate attacks, because the outcomes terrorists want to see will not be possible and inadvertent introductions will be eliminated with scarcely a footprint.30
Thirteen years later, the U.S. government has made some notable commitments to countering the threat to animals. For example, the National Animal Health Laboratory Network (NAHLN) works to detect biological threats to food animals, although its funding is not as robust as its human-health counterpart, the Laboratory Response Network for Bioterrorism. DHS is spending $1.25 billion dollars to build a modern animal disease laboratory in Manhattan, Kansas (to replace PIADC). At the border, U.S. Customs and Border Protection agricultural inspectors work daily to prevent the import of food and agricultural products that could harm human health, animal health, and the economy. USDA inspectors and veterinarians similarly safeguard the food supply through border-based health inspection and quarantine of incoming animals, and the USDA Food Safety and Inspection Service and the U.S. Food and Drug Administration (FDA) safeguard food safety at processing plants throughout the United States and globally. USDA also accredits and trains private-sector veterinarians to detect and respond to disease outbreaks. These and other efforts account for a large portion of the federal investment in defending U.S. food and agriculture.
Yet in context, the F&A sector receives far less attention than many other critical infrastructure sectors. This sector continues to be highly vulnerable, and many of the tools and technologies described by Breeze remain poorly developed and integrated into suitable plans and proper response operations.
Further, many farms are open systems, and biosecurity varies from one farm to the next, a point clearly illustrated during the 2015 HPAI outbreak. As the Government Accountability Office (GAO) found in an analysis of USDA efforts to combat avian influenza, poultry producers and growers oftentimes did not adhere to basic biosecurity practices before and during the outbreak, which resulted in further infection. The USDA relies on poultry producers and contractors to voluntarily take preventive steps to protect their flocks from disease.31 In early 2016, USDA took the first steps to address this issue by publishing an interim rule making indemnity payments contingent on poultry and egg producers and growers certifying their adherence to a biosecurity plan. The rule is limited to large-scale operations for certain animals, and is particularly focused on HPAI. Biosecurity provisions have also been added to the National Poultry Improvement Plan, a voluntary program under which producers can be certified as disease-free for trade purposes.
Thus, the production of food presents what amounts to a chain of vulnerabilities. The intentional disruption of any of the goods and services that comprise F&A could occur at myriad nodes along this chain. Weaknesses of these types put human health, animal health, and the entire agricultural-based economy at risk.
According to GAO, the President’s Fiscal Year (FY) 2015 $23 billion budget request for USDA included only $287 million for animal health efforts – that is, 1.2%.32 While this figure does not include use of the Commodity Credit Corporation (CCC) for response efforts, the dollar value of which can be substantial, the annually appropriated level is simply too low to preventively safeguard animal health to optimal levels. This is a department whose earliest and groundbreaking successes in the nineteenth century were for the proactive protection of animal health. Notable priorities for that nascent department, established by President Abraham Lincoln, included funding the study, control, and eradication of infectious diseases like contagious bovine pleuropneumonia and Texas cattle fever. DHS has invested research dollars at PIADC for FMD vaccines, and construction dollars for the new National Bio- and Agrodefense Facility (NBAF). Yet the President’s FY 2018 request disregards agriculture research and development funding support at DHS, eliminating all of its research programs at PIADC.
Many of the activities in which DHS, USDA, and interagency partners engage are indispensable elements for the development of effective biosurveillance, medical countermeasures (MCM), response capacity, and all other features of effective agrodefense. It is difficult to account for the ways in which these and other expenditures work together to reduce the threat to agriculture and to determine the areas where resources are most necessary. While the forthcoming National Biodefense Strategy should partially solve this problem, an Office of Management and Budget (OMB) assessment of program productivity and return on investment – and one made publicly available – is still needed.
Leadership
The ownership of F&A by the private sector and the significant contribution it makes to SLTT economies necessitates significant federal interaction and collaboration with non-federal stakeholders. Presidential Policy Directive 21 designated the USDA and the Department of Health and Human Services (HHS, delegated to the FDA) as the federal agencies to lead the infrastructure protection components of the F&A sector.33 Like many of its critical infrastructure counterparts, the complexity of facilitating resilience within this sector necessitates significant involvement by other federal departments and agencies, as well as with the non-federal parties that own and operate it. The Commission has previously stated that political-level leadership at the White House is needed to drive priorities for biodefense, and this by extension includes agrodefense, particularly in light of policy and political divisions outlined in this report.
Federal Structural Organization
The defense of U.S. agriculture is a broad and intricate mission space, its complexity reflected in the biodefense enterprise writ large. USDA and FDA have primary federal responsibility for encouraging the national security of agriculture. The USDA Office of Homeland Security and Emergency Coordination provides the primary means of communication between USDA and other departments at a policy level. Most other federal departments and agencies also help to protect this sector, with DHS serving a leading role in addressing national security related incidents.
The functions necessary to do this include intelligence analysis, law enforcement, animal health, plant health, public health, environmental remediation, and outbreak response and recovery. The 2008 Food and Agriculture Incident Annex (FAIA) to the National Response Framework, which addresses only the response and recovery element of agrodefense, lists USDA and HHS as Coordinating Agencies, and the Department of Commerce (DOC), the Department of Defense (DOD), the Department of Energy, DHS, the Department of Interior (DOI), the Department of Justice (DOJ), the Department of Labor, the Department of State, the Department of Transportation, the Environmental Protection Agency (EPA), the General Services Administration, the U.S. Agency for International Development (USAID), the U.S. Postal Service, and the American Red Cross as Cooperating Agencies.34 The forthcoming update to the FAIA (expected in 2017) will provide further specificity, naming subordinate agencies and offices within many of these departments, and detailing how agencies should coordinate with one another.
Ultimately, the United States Code (7 USC 8310(e)(2)) designates the USDA as the lead agency with respect to issues related to pests and diseases of livestock; 7 USC 7652 likewise designates the Secretary of Agriculture as the principal federal official responsible for coordinating all federal research and extension activities related to food and agricultural sciences. However, like other areas of biodefense, federal responsibilities for agrodefense are by necessity spread broadly across the interagency. Roles and responsibilities under the U.S. Code and other authorities are not necessarily coordinated, nor are the authorities always exercised in a way that has prioritized needed activity. White House-level leadership is, therefore, critical to minimize overlap, identify mission gaps, and coordinate effort. The Commission has recommended previously that the Vice President serve in this role.
SLTT leadership at the political level is no less fundamental to all phases of protecting animal agriculture. In January 2016, when avian influenza appeared in Indiana, then-Governor Michael R. Pence was the first high-level state official to arrive at the emergency command post in Jasper, Indiana. Governor Pence’s appearance motivated both officials and producers to act quickly and prevent this outbreak from spreading as far as it had during the national outbreak in 2015. According to Dr. Bret Marsh, Indiana State Veterinarian:
He was there first. And it frustrated some of the press because they didn’t know he was coming. But he didn’t want to be the event. He wanted the people to complete the event and keep their work moving forward. And I would get these text messages from some guy named Mike… I’ve worked for several governors, but I’ve never had text messages… So I think, from the Vice President’s office, clearly he has an understanding and understands the importance of these issues, in our state, and, therefore, across the country.35
Dr. Marsh also believes that without local collaboration, the outbreak would have spread farther. Producers, not officials, culled poultry at affected farms, realizing that it was “the right thing to do.” Additional SLTT interventions are needed to strengthen government partnerships with industry, build expertise, and develop response plans before outbreaks occur.
While the Commission emphasizes in this and in prior reports that two high levels of leadership are necessary to identify appropriate political direction and policy development and coordination, the Commission also reinforces the need for operational leadership during crises as the third critical piece. Congress should consider evaluating the response planning and recovery elements of Homeland Security Presidential Directive 9 (HSPD-9), particularly those areas that pertain to response capabilities and F&A-specific response plans to ensure that they meet National Preparedness System requirements. The forthcoming issuance of an updated National Food and Agricultural Incident Annex (see Coordination chapter) provides a timely opportunity to do so.
Artificial Policy Divisions Hamper Progress
A complex web of ecological interactions governs the spread of infectious disease. All efforts to prevent and plan for biological events impacting humans must therefore integrate with animal and environmental health initiatives. Animals can be susceptible to many of the same threats as humans and they can also act as conduits for human infection. Further, animals can be terrorist targets in their own right. All agrodefense efforts must integrate human, animal, plant, and environmental health elements into decision-making, budgeting, and operations.
Assessment and reduction of risk to the F&A sector have been led primarily by DHS, USDA, and FDA. HSPD-9 and the F&A Sector-Specific Plan (part of the National Infrastructure Protection Plan) provide a foundation for the protection of this sector.36 However, associated efforts to prevent, deter, prepare, detect, attribute, decontaminate, remediate, and mitigate agricultural events are not well integrated. Additionally, medical and other countermeasures to protect animals and plants are unavailable for most emerging pathogens. Further, the Bioterrorism Risk Assessment process conducted by DHS appears to be insufficiently linked to follow-on investments that could mitigate this problem via risk management activities.
Optimal biodefense can only be achieved when grounded in an ecological understanding of the entire health picture. The distributed nature of health-related responsibilities across the federal government creates bureaucratic silos that often fail to recognize the interrelatedness of human, animal, plant, and environmental health. A designated leader at the White House who recognizes this interconnectedness could drive integration across federal efforts.
Recognition of the Threat by High-Level Leadership
In 1999, Congress established the Advisory Panel to Assess Domestic Response Capabilities for Terrorism Involving Weapons of Mass Destruction, also known as the Gilmore Commission. This Commission produced several reports for the President and Congress, the first of which noted that agriculture was a highly vulnerable sector and that the biological threat to it deserved more attention than it was getting at the time.37
Since then, White House councils (e.g., Domestic Policy Council (DPC), National Economic Council (NEC), Homeland Security Council (HSC), and the National Security Council (NSC)) and the Office of Science and Technology Policy (OSTP) have taken up the issue of agrodefense in various ways. Under the direction of President George W. Bush, White House staff evaluated the extent to which the nation had secured F&A critical infrastructure sector and related sectors and activities. President Bush’s HSC identified agrodefense as a pressing concern, and began developing a presidential directive to address it as a part of biodefense. However, the enormity of the risk to agriculture, as well as the precedence of deep-seated and long-standing turf protection among the departments and agencies, drove the Bush Administration to separate agrodefense from other biodefense efforts. The White House subsequently produced two directives in 2004: HSPD-9, Defense of United States Agriculture and Food38 and HSPD-10, Biodefense for the 21st Century.39 These were written separately, although the staffs were the same, and there was cross-over of ideas and an acknowledgement of the realities of One Health. But there were also deep-rooted turf issues that manifested during the process, reflecting the same territoriality seen throughout the federal government today.
Congress also recognized the threat to the sector and sought to address it through oversight and legislation. Senator Pat Roberts convened the first congressional agroterrorism hearing in 1999.40 More oversight followed. The decision to build the NBAF resulted in hearings and legislation about the national need for agrodefense research and response capability and capacity. The 2014–15 avian influenza outbreak drew attention to the flaws in agrosecurity, and both the House Committee on Agriculture and the Senate Committee on Agriculture, Nutrition, and Forestry held hearings to identify systemic shortcomings in the response to that outbreak.41 Both chambers of Congress heard from witnesses who identified biosecurity measures that could be legislated, including a mandatory disease prevention program and an FMD vaccine bank.42 In addition, the House Committee on Agriculture held a hearing on the FMD threat,43 and the House Committee on Homeland Security held hearings on agrodefense more broadly.44 Congress tasked GAO in the first decade of the 2000s to conduct a variety of studies regarding protection of the F&A sector; since 2010, congressional requests have been few and usually in response to – not in advance of – outbreaks affecting agriculture.
As a reflection of federal interest in agrodefense, the NBAF deserves special mention. The NBAF is part of the USDA and DHS “plan to provide safe, secure, and state-of-the-art agricultural biocontainment laboratories that research and develop diagnostic capabilities for foreign animal and zoonotic diseases” called for by HSPD-9.45 The Executive and Legislative Branches have supported the creation of the NBAF, if haltingly, while working through controversies. The overall trajectory of support to build this laboratory has demonstrated a federal commitment to agrodefense research and response. DHS, with substantial contributions from the state of Kansas and the city of Manhattan, Kansas, will spend well over $1 billion to develop it.
All of this oversight and commitment, and the areas that have lagged or been omitted from it as described in this report, are occurring in the absence of a national strategy and corresponding implementation plan. As described in the Blueprint for Biodefense, the nation requires a comprehensive National Biodefense Strategy that integrates the input of all non-federal stakeholder groups. Congress has acted upon the Commission’s recommendation and required the development of this Strategy per Section 1086 of the National Defense Authorization Act for FY 2017 (Public Law 114-328). While the Commission recommended that the Vice President take charge of producing this Strategy, Congress directed four departments, DOD, DHS, HHS, and USDA, to work together to do so. The drafters in the House and Senate Committees on Armed Services included USDA because they recognized the integral role of agriculture in our biological security and the serious threats to this sector.
In accordance with Recommendation 3 of the Blueprint for Biodefense to develop, implement, and update a comprehensive National Biodefense Strategy:
The White House must ensure that the National Biodefense Strategy (Strategy) and implementation plan address threats to food and agriculture. As part of this process, the National Security Council, Domestic Policy Council, and National Economic Council, in consultation with the Secretaries of Agriculture, Defense, Health and Human Services, and Homeland Security, should jointly review Homeland Security Presidential Directive 9, Defense of United States Agriculture and Food, determine where it falls short in addressing today’s agrodefense needs, and incorporate updates into the Strategy and its implementation plan. While leadership and policy coordination of interagency federal activity should be centralized, responsibilities for agrodefense will continue to be distributed nationally. The Strategy must recognize this decentralized nature of the U.S. food and agriculture critical infrastructure sector.
USDA has made some critical investments in agrodefense, such as directing research efforts at PIADC with significant emphasis on FMD vaccine, providing food and agrodefense grants through the National Institute of Food and Agriculture, and working with the FBI, FDA, and other agencies to conduct law enforcement and public health investigations. USDA, with some White House direction, also produced a number of policy documents. In addition to USDA, the DOC, DOI, and various HHS agencies (e.g., CDC, FDA), have generated relevant F&A policy documents. While these departments and agencies all take some responsibility for agrodefense, USDA and FDA are ultimately responsible. In addition to DHS input, USDA leadership and FDA leadership must make National Biodefense Strategy contributions a top priority.
While policies and plans are important, they will mean little without an agency to own them and dollars to implement and exercise them. And yet, a federal fiscal commitment to agrodefense is not entirely apparent. The Homeland Security Act of 2002 (HSA) required that the President’s budget request incorporate a homeland security funding analysis – in essence, a kind of budgetary cross-cut. According to the FY 2017 analysis, 29 agency budgets included federal homeland security funding across 17 functional areas. The agriculture function accounted for only 0.76% of the total.46
Published not long after the HSA, HSPD-9 also acknowledged the pressing need for budget coordination: “For all future budgets, the Secretaries of Agriculture, Health and Human Services, and Homeland Security shall submit to the Director of the Office of Management and Budget, concurrent with their budget submissions, an integrated budget plan for defense of the United States food system.”47 OMB did collect this information and included it in the annual homeland security analysis in accordance with the HSA, but this analysis was high level and did not provide any detail regarding the expenditures in the functional areas. Furthermore, Congress eliminated the reporting requirement altogether in its FY 2017 appropriations law. The Commission strongly recommends statutory reinstatement of the analysis and continued collection of this information on the part of OMB.
In accordance with Recommendation 4 of the Blueprint for Biodefense to develop a unified biodefense budget aligned with the national biodefense strategy, the Commission proposes the following:
The President and congressional appropriators should ensure that detailed agrodefense expenditures are identified and included in the recommended data call for and development of a crosscutting biodefense budget analysis. These requested expenditures should be accompanied by impact evaluations. Any gaps recognized as a result should be addressed in the National Biodefense Strategy.
Coordination
Many federal departments and agencies share responsibility for agrodefense. Coordination of these efforts is paramount. Because agricultural outbreaks may result from natural events or from deliberate actions, coordination between animal health and law enforcement is particularly critical. The health mission of the USDA and the investigative mission of the FBI must be jointly acknowledged, exercised, and implemented.
Law Enforcement and Attribution of Attacks
According to the FBI, the intentional introduction of disease is difficult to differentiate from accidental or naturally occurring outbreaks.48 Authorities for animal health, plant health, and law enforcement must work with one another from the earliest stages of an outbreak to attribute its source. Some of the most important elements of this joint cooperation include rapid notification of agreed-upon triggers, early threat reports, and unusual disease events, as well as efficient criminal-epidemiological investigation and response. Yet there has been an inconsistent recognition that agriculture is a target of domestic and international terrorist elements, and that intentional means of introduction should be equally considered when suspicious or unusual animal-plant disease events and other recognized triggers are initially detected. Continued training such as that provided by the FBI through its Criminal and Epidemiological Investigation course will help support better understanding between the agriculture and law enforcement communities, help the investigation of threats to animals and plants, facilitate threat and operational awareness, develop information sharing protocols, and foster SLTT health-law enforcement contact networks. Additionally, broad distribution throughout the food and agricultural community of resources developed jointly by USDA, FBI, and FDA, such as the Criminal Investigation Handbook for Agroterrorism, will help increase awareness of the threats to F&A and how these communities can work together to investigate outbreaks in, and suspected acts of terrorism against, this sector.
When this report went to press, federal partners were drafting a revised FAIA that would provide updated and more comprehensive guidance for federal interagency planning efforts involving food and agricultural incidents. The development of an updated annex is a critical step toward improved agricultural event preparedness, and ideally the final version will contain more in-depth detail on the roles and responsibilities assigned to the federal interagency than the 2008 version. Challenges in developing the revision in a way that prioritizes both natural and intentional events may reflect a central issue about the perception of agricultural terrorism. Law enforcement investigation of terrorism is well within the scope of the FAIA’s purpose – interagency planning and coordination for response and recovery. Much as the recently-updated Nuclear/Radiological Incident Annex establishes a clearly-defined role for the Bureau’s investigatory responsibilities in the aftermath of weapons of mass destruction (WMD) terrorist acts,49 discussion of the details and parameters of FBI and other law enforcement response must be included in the response to F&A events.
Scenarios detailed in any new FAIA should include intentional introductions of food and agricultural pests or contaminants, and should address the source and means of those introductions. The FBI considers any foreign animal disease outbreak suspicious until proven otherwise, and seamless coordination in the early stages of investigation among law enforcement, animal health, and public health is therefore critical. Mitigating animal health impacts indeed must be the priority, but there is no reason that protocols developed by the FBI cannot be leveraged to ensure a concomitant investigation to determine the source of the outbreak which, if intentional, must be known quickly to then disrupt follow-on acts of terror or crime.
In accordance with Recommendation 9 of the Blueprint for Biodefense to better support and inform decisions based on attribution of biological events, the Commission proposes the following:
The Administrator of the Federal Emergency Management Agency should coordinate with the Administrator of the Animal and Plant Health Inspection Service (APHIS) and the Director of the Federal Bureau of Investigation to ensure that any update of the Food and Agriculture Incident Annex (Annex) recognizes and addresses the investigative mission of the Federal Bureau of Investigation (FBI), and clearly directs other federal departments and agencies to support inquiries into suspected acts of agricultural crime and terrorism. The next iteration of the Annex should incorporate concepts of initial consideration of intentional threats in unusual or suspicious disease events; the roles and responsibilities of the FBI, USDA Office of Inspector General, and FDA Office of Criminal Investigations; and subsequent joint criminal-epidemiological investigations. The Annex should also enumerate the role played by the nation’s fusion centers in coordinating and disseminating information.
Further, the aforementioned officials should ensure that, to the greatest extent possible, responsibilities in this Annex related to law enforcement inquiries or investigations of acts of agricultural terrorism align with similar activities in the Nuclear/Radiological Incident Annex, the Biological Incident Annex, and any other incident annex to the Response and Recovery Federal Interagency Operational Plans.
The Administrator of the Federal Emergency Management Agency should coordinate with the Administrator of APHIS and the Director of the FBI to ensure that Annex updates would be required to be regularly exercised at least at the state level, as is done with other areas of national security. These exercises should provide a means for the named agencies, as well as other federal and non-federal partners, to develop measurements of the capabilities needed for adequate and economically justifiable response and recovery efforts. They should also be used to gauge the value of funding programs to enhance the capabilities described within the Annex.
Collaboration
Collaborative effort within the interagency and among non-federal stakeholders has been a cornerstone of homeland security efforts since September 11, 2001. That same collaborative effort is necessary within agrodefense. This section of the report focuses on challenges in two areas: biosurveillance and reporting/information sharing. Avian influenza and other outbreaks have demonstrated the critical importance of timely and accurate biosurveillance. Early detection is one of the best methods available to prevent the spread of infectious disease. The emergence of infections not just in rural but also in urban areas, as evidenced by a rare avian influenza strain that infected 500 cats (and at least one human) in a New York City animal shelter in late 2016, demonstrates a requirement for vigilance and an acknowledgement that all areas, rural and urban, and many species, wild and otherwise, must be part of any surveillance framework. Adequately funding data collection and establishing a nationally notifiable animal disease list are critical to the success of this system, as is reporting and information sharing among federal, SLTT, and private sector stakeholders. Ultimately, leadership over federal biosurveillance efforts and, in particular, the integration of these efforts is still needed.
Biosurveillance
The early detection of infectious disease outbreaks is one of the most important means available to mitigate their impacts and shorten the duration of response. This detection should occur at the level of livestock production and in wildlife. Stakeholders in this area span from government agencies at all levels to local farmers, veterinary hospitals, and even poison control centers. Although the control of many diseases is not possible in wildlife, early detection is one of the best defenses against catastrophic impacts of agricultural and zoonotic disease threats.
The drafters of HSPD-9 understood this concept. HSPD-9 tasks DOI, USDA, and EPA to operate surveillance and monitoring systems (section 8); DOJ, DHS, and the intelligence community (IC) with intelligence collection and analysis (section 9); and DHS with integration of this information (section 10). Each of these elements exists in various stages of maturity and interagency integration. An important missing element is a standard of expectation or quality by which the value of investment in biosurveillance can be measured. Such a standard could include: the key area of characterization; risk determination; potential course of action; and a means of assessing the value of the contribution these measures have on health. Such a standard does not currently exist in biosurveillance, and without it, funding will continue to be inhibited and uninformed.
In December 2014, the USDA identified HPAI in poultry in Oregon and Washington in an outbreak that ultimately reached 232 farms across 21 states before federal and state officials and industry partners eradicated it.50 The federal government spent $879 million to contain the outbreak,51 a figure that includes $610 million toward response activities, $200 million in indemnity payments, $34 million in planning costs for the coming autumn, and $35 million in overtime, travel, and supplies for USDA employees.52 While the costly response prevented a larger disaster, the 2014–15 outbreak still cost the U.S. economy $3.3 billion.53 Nearly 7.5 million turkeys, 43 million layer hens, and 3.5 million replacement pullets (young female hens) were destroyed,54,55 and an estimated 15,000 jobs were lost in the egg industry.56 Indirect costs included higher prices for eggs;57non-indemnified losses to producers (estimated at more than $1 billion);58 and bans placed by 15 countries on poultry imports from the United States, with many other countries placing targeted bans on particular U.S. states or regions.59
In January 2016, an unrelated HPAI strain appeared in a commercial turkey flock in Indiana, and a low pathogenic strain was confirmed at eight nearby farms; approximately 414,000 birds were depopulated to control this outbreak which lasted until May of that year.60
Combined, these avian influenza outbreaks resulted in the death or culling (selective slaughter) of 50.6 million animals, cost the federal government $930 million, and cost the U.S. turkey and egg sectors $1.6 billion.61 Indirect impacts on the U.S. economy were even higher. We can expect more events of this nature in the years to come. As recently as March 2017, another HPAI outbreak occurred, this time in Tennessee.
The 2014–17 U.S. avian influenza outbreaks exemplify a partially effective detection and surveillance capacity linked to a response capacity fraught with significant challenges. The GAO reported that USDA evaluated response weaknesses revealed by the first two outbreaks (2014–15 and 2016).62 USDA identified challenges in biosecurity, continuity of business planning, diagnostic testing, epidemiological investigation, incident management, mass depopulation and euthanasia, biosurveillance, and vaccination, among other categories. While response capacity is clearly of significant importance given the inherent difficulty of preventing pathogens like HPAI from entering U.S. borders via wild birds, some increased emphasis on biodetection and biosurveillance in wildlife and livestock could improve mitigation efforts toward avian influenza and other diseases. This is particularly true for wild bird surveillance, which requires steady funding in advance of outbreaks.
Rapid biodetection, diagnosis, and integrated biosurveillance remain critical functions toward which the nation has made great strides, yet which still lag behind the need. Biodetection is hampered by an insufficient focus on rapid pen-side diagnostics, and insufficient investment to develop new wildlife disease detection technologies and validate existing tests (e.g., PCR assays for avian influenza and other pathogens). Biosurveillance is perpetually challenged by information sharing problems. HSPD-10 described the need for “an integrated and comprehensive attack warning system to rapidly recognize and characterize the dispersal of biological agents in human and animal populations, food, water, agriculture, and the environment.”63 However, animal health surveillance remains somewhat segregated from the model of comprehensive biosurveillance described. Livestock health surveillance is currently performed for the benefit of agriculture and food animal production. These data are typically unavailable on a regular basis to federal agencies with surveillance responsibilities outside of the USDA, although reportable zoonoses do make their way to state and federal public health authorities. Some argue anecdotally that animal and human health surveillance data are insufficiently integrated; while this may be the case, the Panel has to-date identified few examples that any such lack of integration has directly caused negative health impacts in animals or people. A deep evaluation of the nodes of connectedness, the lack thereof, and case studies of where failures have occurred could help guide further biosurveillance policy.
Spurred by outbreaks of FMD and BSE in the United Kingdom, along with the spread of West Nile virus in the United States, the USDA established the National Wildlife Disease Program (NWDP) in FY 2003 to provide wildlife disease surveillance and management at a national level. Because state wildlife agency efforts tend toward wildlife management rather than disease diagnosis, understanding of the wildlife disease surveillance picture, particularly in the context of the broader animal and human health picture, has fallen to the federal government. The NWDP program is designed to reveal key features of infectious diseases, such as prevalence, species predilections, species reservoirs, predominant strains, and geographic scope of given pathogens. The program accomplishes a great deal despite its low level of appropriated funding. For instance, NWDP instituted national disease monitoring programs for swine brucellosis, pseudorabies, and classical swine fever.64 The program also undertook a pilot study examining feral swine as sentinels for anthrax.65 Anthrax and other material threats are targets of other NWDP initiatives, such as its efforts to sample wildlife species for the presence of tularemia and plague. The monitoring was put to use in Indiana after the 2016 avian influenza outbreaks to sample mice, starlings, gulls, and other animals that might be harboring the offending virus.66 USDA also funded wild bird surveillance through its CCC funds; the USDA funding allotments toward surveillance are shared with partner agencies, an important example of collaboration.
Initially funded at approximately $6.2 million, NWDP has not seen an increase since its inception and operates now at just under $4 million. This fact illustrates that each year for the last decade-and-a-half the operational side of the program has ended up with about $3 million to surveil for more than 75 pathogens, toxins, and syndromes, at multiple scales ranging from state to national, continental, or even international.
This figure is surprisingly low when placed in context. USAID’s EPT PREDICT, a critical global wild animal surveillance program, receives roughly $20 million annually; yet the core domestic program designed for wildlife sampling receives one-fifth of that. While the United States is not considered a hotspot for emerging infectious disease, its land mass, biodiversity, and commercial agricultural sector create a trifecta of risk for pathogen introduction. The surveillance effort should be commensurate with that risk. Much of the international biosurveillance work undertaken by USAID, particularly in predictive efforts, may serve as a model for future surveillance programs, and its work to build capacity abroad should be reflected as an element in the National Biodefense Strategy.
Current funding levels present limitations to our situational awareness and accumulation of scientific knowledge. As stated by Bevins et al., “Large-scale surveillance programs such as this… are important for providing ecological data on infections at politically and biologically relevant scales.”67
Congress continues to appropriate funding as particular events occur. From 2006–11, USDA, DOI, and SLTT agencies implemented an NSC-requested plan for a nationally coordinated avian influenza surveillance effort in wild birds.68,69,70 Their funding came from separate appropriations to the two federal departments as per the standard congressional approach, one that does not incentivize inter-departmental cooperation unless the subcommittees jointly build such partnering into the law. White House direction was likely, therefore, an important element of the program’s ultimate success. Efforts ceased in 2011, and were not renewed until 2014 when HPAI reappeared in U.S. commercial poultry flocks. If history repeats itself, USDA or Congress may discontinue the program once again when a lull in avian influenza outbreaks tempts them to turn their funding elsewhere.
The integration of collected surveillance information is an essential component of the process.71 Yet this piece has been perhaps the one most stymied by bureaucracy. The subject of a national, comprehensive, and integrated human and animal health surveillance system has been much discussed since the issuance of HSPD-9, which stated:
The Secretary of Homeland Security shall coordinate with the Secretaries of Agriculture, Health and Human Services, and the Administrator of the Environmental Protection Agency, and the heads of other appropriate Federal departments and agencies to create a new biological threat awareness capacity that will enhance detection and characterization of an attack. This new capacity will build upon the improved and upgraded surveillance systems described in paragraph 8 and integrate and analyze domestic and international surveillance and monitoring data collected from human health, animal health, plant health, food, and water quality systems.72
Similar to the related requirement in HSPD-10, no such system has ever been implemented. DHS’ National Biosurveillance Integration System (NBIS) might have achieved this goal, at least in part, but has not realized the function envisioned for it for reasons described in the Blueprint for Biodefense. Acquiring the necessary data has proven to be difficult. Much of the data are owned by the private sector, thus requiring protected information policies that incentivize sharing. Similarly, successful analysis to detect emerging health threats depends on the cooperation of federal and state agencies. Despite such challenges, the Panel has previously concluded that NBIS could have been successful with centralized stewardship; and it remains true that White House leadership could still provide the basis for the coordination and collaboration necessary to optimize the function, if not the NBIS itself. Should NBIS be expected to continue its mission, the White House must get behind and support it. The White House would need to direct interagency sharing of information for the system, and encourage other departments to not just provide information, but to seek information from NBIS through well-formed queries with stated purpose for use. NBIS in turn should be required to evaluate how well its information contributions to DHS and other departments assist in risk reduction and other desired impacts associated with integrated biosurveillance. The approach should be tied to the standards for biosurveillance discussed previously.
The implementing partners of the wild bird surveillance system established an interagency steering committee for surveillance of influenza in wild birds. USDA APHIS (Wildlife Services and Veterinary Services), the U.S. Geologic Survey (DOI), U.S. Fish and Wildlife Service, CDC, state representatives, and the National Flyway Council are members of this Interagency Steering Committee for Surveillance for HPAI in Wild Birds. This committee has produced interagency plans for detection of HPAI in wild birds.73 The steering committee has been a cohesive unit for designing and implementing large scale surveillance systems. The development of more interagency steering committees similar to that for HPAI could perhaps provide a platform for this kind of education, information sharing, and relationship building.
The NAHLN, a network of federally-supported partner labs located across has country, also serves a vital function in quickly identifying, confirming, and providing diagnostic surge support for infectious disease outbreaks. In the 2014 Farm Bill, funding was authorized at the level of $15 million annually. The 2018 Farm Bill provides an opportunity for Congress to consider whether the currently authorized level is sufficient to meet the growing need for a national system capable of handling its daily diagnostic demand as well as surge demand for a massive outbreak. Additionally, in the 2008 Farm Bill, Congress authorized the creation of a prevention program for plant diseases and disasters funded by the CCC.74 Congress should consider establishing a fund to address similar programs for animal health, one that provides more robust support for early detection and surveillance efforts at the state level.
In accordance with Recommendation 14 of the Blueprint for Biodefense to improve surveillance of and planning for animal and zoonotic outbreaks, the Commission proposes the following:
The National Security Council should direct interagency partners to develop a standard of expectation or quality by which the value of investment in biosurveillance can be measured. The White House should consider the full scope of wildlife surveillance activity that would benefit wildlife, livestock, and human health, and develop a commensurate budget request. The Administration and Congress should commit to such a plan for the long term. Congress should fund and facilitate enhanced opportunities for data collection from livestock and wildlife by the Department of Agriculture (USDA), Department of Homeland Security (DHS), and Department of Interior, through increased appropriations to the USDA National Wildlife Disease Program. The Secretary of Homeland Security should further DHS collaboration with other federal, state, local, tribal, and territorial, and private sector entities that collect animal health data. Congress should assess whether DHS and the USDA have the needed authorities to ensure the effective sharing of information, and amend statute as necessary.
Congress should continue to fund the National Animal Health Laboratory Network in FY2018 and thereafter at no less than authorized levels, leaving open the possibility that additional funds may be required to fulfill the Network’s mission as the need to rapidly diagnose outbreaks grows.
Congress should establish a prevention fund for animal health disease and disaster programs through which capability gaps identified in this report and other relevant agrodefense analysis can be addressed. The Commodity Credit Corporation would be an appropriate vehicle for this funding. This fund could be based on the program created for plant health in Section 10201 of the Food, Conservation and Energy Act of 2008.
Reporting and Information Sharing
The SECD outbreak, perhaps more than any other livestock infectious disease outbreak in recent memory, demonstrated the importance of early reporting, whether for foreign or endemic diseases. APHIS has developed a National List of Reportable Animal Diseases (NLRAD), which has two categories: Notifiable Diseases and Conditions, and Monitored Diseases. The Notifiable Diseases and Conditions consists of foreign animal diseases, emerging disease incidents, and regulated disease incidents. Currently, only accredited veterinarians are required to report specific diseases, such as foreign animal diseases and other diseases not known to exist in the United States.75
Monitored diseases do not have a requirement for immediate reporting; they are included only in a monthly reporting requirement by state animal health officials and only when confirmed (not at the suspected or presumptive stage). Furthermore, disease reporting rules for monitored diseases do not require states to report the specific number of cases that have been identified. Last year, only 36 states voluntarily reported diseases on this list to USDA. Furthermore, some states have their own unique reportable disease lists which often differ in terms of which diseases are reported (e.g., the only virus present on all state lists is influenza). Though newly-identified emerging infectious diseases are often placed on the mandatory notifiable reporting list, many known, long-standing diseases that are on the voluntary monitored list have not historically been tracked reliably or consistently.
A systematic and comprehensive animal disease reporting system that codifies reporting requirements and provides for consistent reporting is needed. The 2013 swine coronavirus outbreaks demonstrate the disadvantages apparent from the lack of such a system. Although USDA was aware of the initial cases, it did not take further regulatory action that would require reporting from affected farms over concerns that it could have negative impacts on the swine industry. Instead, USDA initially supported industry-led efforts to address the outbreaks.76 A balance between restrictive reporting requirements and the ability of industry and states to manage their own agricultural affairs is needed. The goal should be to allow greater availability of information, coordination of effort, quicker response, and reduced impacts on all stakeholders. The foundation for this eventual outcome is in place: many states are already voluntarily working with USDA to report diseases, and further support through the NAHLN, cooperative agreements, and veterinary accreditation can help strengthen regular reporting of diseases at the state level.
A 2014 concept paper from the USDA on building a reportable disease system has yet to be implemented, although the USDA has since issued a follow-on publication, a framework designed as a pre-cursor to rulemaking. USDA states that, “Regulatory action will officially recognize the NLRAD and codify specific reporting requirements for State animal health officials, laboratory personnel, veterinarians, producers, and others. The U.S. agriculture infrastructure is vulnerable to significant damage from listed as well as emerging diseases.”77 The NLRAD will provide consistent reporting across the United States and help animal health officials protect the U.S. agriculture infrastructure. USDA posted the draft framework for public comment in late 2016; if implemented in regulation, it would make reporting of notifiable diseases mandatory by veterinary practitioners, producers, diagnostic laboratory personnel, and others with knowledge of real or suspected occurrence of these notifiable disease categories. Monitored diseases are to be reported on a monthly mandatory basis by state animal health officials. Additionally, for the first time, private laboratories and entities would be required to report both notifiable and monitored diseases. Notably, the framework would rely on collaboration between federal, state and industry officials to decide the detail of data needed for each disease on the monitored list. At the time this report went to press, the framework was in a review period after receiving public comments.
In accordance with Recommendation 7 of the Blueprint for Biodefense to integrate animal health and one health approaches into biodefense strategies, the Commission proposes the following:
The Administrator of the Animal and Plant Health Inspection Service (APHIS) should finalize the rule to establish the National List of Reportable Animal Diseases (NLRAD), in accordance with APHIS’ proposed framework and stakeholder comment on that framework. Once finalized, the Administrator of APHIS should ensure that sufficient data systems are in place to properly support the reporting and dissemination of data through the NLRAD. Additionally, the Administrator of APHIS should take appropriate steps to encourage and incentivize rigorous reporting from laboratories, veterinarians, and other stakeholders for cases of diseases on the monitored list, beyond the requirements detailed in the proposed framework.
Innovation
Innovative thinking, both in how we govern and in the technological solutions we bring to defense challenges, has been one of the foremost messages of this Commission. The nation needs new ideas and new scientific solutions to push agrodefense approaches beyond their current limitations. Options beyond culling, particularly those that consider animal welfare, must become core tenets of our response; government incentives for innovative research where commercial markets are lacking must become the norm; and academia, producers, and government officials must be encouraged to work together in new ways.
Next-Generation Medical Countermeasures
As important as biosurveillance is, the bigger challenges seem to rest with other elements of the system: we have minimal MCM stockpiles or agreements with vendors; we lack the capability to produce MCM on demand; we cull animals because it is deemed to be the only option; and the direct and indirect costs of response are enormous. Reasons for this vary from insufficient federal investment in innovative technologies to the logistical hurdles, cost, and trade ramifications of vaccinate-to-live control strategies.
HSPD-9 requires a coordinated federal effort, led by the Secretary of Homeland Security, to accelerate and expand the development of countermeasures against catastrophic animal, plant, and zoonotic diseases. Relatedly, HSPD-9 requires DHS, HHS, USDA, and EPA to develop a National Veterinary Stockpile (NVS). The White House envisioned the stockpile to contain “sufficient amounts of animal vaccine, antiviral, or therapeutic products to appropriately respond to the most damaging animal diseases affecting human health and the economy and that will be capable of deployment within 24 hours of an outbreak.”78 To date, the NVS has not been authorized in statute.
While the NVS maintains supplies like personal protective equipment and depopulation equipment which have been distributed and used successfully in recent outbreaks, from an MCM standpoint, the NVS is entirely inadequate. For instance, although the stockpile had 9 million doses of vaccine for a North American avian influenza strain (H5N3) at the time of the 2015 HPAI outbreak, it lacked any doses for the strains that actually were infecting poultry during that outbreak. Following the outbreak, APHIS issued a series of Request for Proposals (RFPs) to stockpile avian influenza vaccine for those strains to be used in future outbreaks. In July 2016, APHIS released its final RFP to acquire an undisclosed number of avian influenza vaccine doses, a purchase that used funding through the CCC. However, without sustained, continued funding, these additional doses will eventually reach the end of their shelf-life and will not be replaced.
Vaccination is generally an effective method of influenza control in poultry.79 Yet vaccination factors only minimally into USDA HPAI plans80, and it is unlikely that the NVS has sufficient access to HPAI vaccine for use in combating any large epidemic. Many elements of vaccination indeed make it a complex technical and policy decision: an abundance of viral strains confounds vaccine formulation and stockpiling decisions; vaccinated animals can still shed virus; and vaccination can negatively impact trade status. Yet mass culling is losing favor among the public and should not be the only option. MCM will need to play a more prominent role, and policy and technology will need to catch up to that necessity.
The lack of vaccine available for use during the 2015 outbreak points to larger problems facing the NVS. While USDA APHIS applies a threat-based approach to vaccine procurement, the agency lacks sufficient funding to procure the MCM that threat-based analysis actually reveals. APHIS is unable to support the procurement of MCM for many of the diseases on its High-Consequence Foreign Animal Disease and Pests list. There are no therapeutics in the stockpile, and mass procurement of vaccines for outbreaks is frequently a reactionary practice. In recent years, the NVS received on average $4 million per year in congressional appropriations, vastly less than that for the Strategic National Stockpile (SNS) which received $575 million in FY 2017 to serve a similar role for human health. While the precise dollar value of an optimal veterinary stockpile is not publicly known, and may not be the same as for the human stockpile, the magnitude of the difference is striking given that many of the costs for development and stockpiling are expected to be similar. At $4 million annually, USDA is forced to find efficiencies in the NVS supply chain and forge outside partnerships just to provide a limited supply and range of countermeasures. The NVS appears to be little more than a vehicle for MCM distribution, rather than an end-use driver for federal identification, procurement, and stockpiling of priority MCM. It is extremely concerning that a funding level that appears to be based on historical precedent rather than risk-based allocation is driving the contents of the nation’s stockpile of veterinary countermeasures. At $4 million, the NVS can only remain on standby and await emergency funding assistance (e.g., borrowing from the CCC), to purchase sufficient amounts of a vaccine during a crisis.
Insufficient federal support for the development of animal vaccines and countermeasures has created an incentive vacuum for the private sector to create them. NVS funding has focused on procuring readily available vaccines, rather than demonstrating a market commitment to procurement the way the BioShield Special Reserve Fund was designed to do for human MCM. Companies often face difficulties in bringing new animal vaccines, antivirals, and therapeutics to market, and those that would develop agricultural countermeasures that lack a commercial market have minimal advanced R&D support and no procurement commitment in the form of robust NVS funding. In the absence of such support, and without the guarantee of a viable federal market, companies hesitate to commit to developing countermeasures at all. Funding the NVS alone is, therefore, insufficient. If the federal government wants to meet the requirements of HSPD-9, a far greater investment in advanced R&D is also necessary. A system of determining how much funding is worth investing in which diseases is therefore of national interest. To date, APHIS has not approved the use of avian influenza vaccines in commercial poultry, including those it has purchased, and it has not indicated whether or when such a determination will be made. The potential of the stockpile will be significantly enhanced through the acquisition of necessary MCM, and through the establishment of policies for their use.
In accordance with Recommendations 27 and 28 of the Blueprint for Biodefense to prioritize innovation and to fully fund and incentivize the medical countermeasure enterprise, the Commission proposes the following:
To meet Homeland Security Presidential Directive 9 (HSPD-9) requirements, the Secretary of Agriculture should assess the ability of the National Veterinary Stockpile to deploy sufficient high-consequence animal disease medical countermeasures within 24 hours. Assessments should prioritize the pathogens identified on the Department of Agriculture’s High-Consequence Foreign Animal Diseases and Pests list. The Department of Agriculture (USDA) should determine the level of funding needed for these efforts, and request it. USDA should use the findings to: inform its budget request; drive federal priorities for medical countermeasure innovation; and incentivize public-private-partnerships to develop, transition, approve, license, and procure these products. Congress should authorize the National Veterinary Stockpile program. Such authorization should require an annual analysis by the USDA of its progress and an identification of persistent capability gaps and costs associated with achieving the HSPD-9 goal.
The Secretary of Agriculture, in consultation with relevant public and private stakeholders, and in alignment with World Organisation for Animal Health policies, should further develop its vaccine use policy for avian influenza and other high-consequence diseases. Vaccine use policy should be based on an underlying commitment by the federal government to respond to outbreaks with rapid diagnostic and vaccine platform technologies.
The NVS also lacks therapeutics and rapid diagnostics. Rapid diagnostics, including patient-side diagnostics, may arguably be the most important element of an animal disease stockpile. They allow for quick decision-making to minimize the spread of disease before it spreads to larger groups, and to prevent inappropriate uses of vaccine or therapeutics. Absent these tools, diagnosis is dependent on empirical observation by veterinarians, followed by time-consuming laboratory identification. The ability to quickly deploy a user-friendly diagnostics capability to the field would allow for a rapid assessment for SLTT animal health officials, enabling earlier decision-making.
The government does not invest sufficiently in pen-side, innovative diagnostic technology, nor even in today’s laboratory-based technology. Diagnostic test kits have short shelf-lives, making them expensive to obtain and maintain. Stockpiling diagnostic test kits would indeed require a sustained financial investment; the need must drive the funding levels, and USDA should determine requirements and request funding in its next budget request to OMB for this purpose.
In accordance with Recommendation 30 of the Blueprint for Biodefense to incentivize development of rapid point-of-care diagnostic technology, the Commission proposes the following:
The Secretary of Agriculture should request adequate resources for the National Veterinary Stockpile to maintain a diagnostic test kit for each stockpiled vaccine sufficient to ensure timely delivery of the kits to laboratories. In the Department of Agriculture’s budget request, the Secretary should request resources to incentivize the development of rapid point-of-care diagnostic devices for high-consequence pathogens.
Among all livestock infectious diseases, the United States has been singularly focused on the development of vaccines for FMD since the 1950s. Yet today, the USDA’s own FMD vaccination strategy states that the United States does not have sufficient vaccine to vaccinate beyond a small focal or moderate regional outbreak.81 The United States contributes funding to the North American FMD Vaccine Bank, which is a repository for vaccine antigen concentrate (VAC). PIADC holds this supply of antigen. Whereas vaccine production from scratch can take up to 14 weeks, industry can produce 2.5 million doses within 21 days with the antigens contained in the bank.82 Yet the supplies in the Vaccine Bank are insufficient to handle a major FMD outbreak in this country. Culling herds continues to be the highly unsatisfactory default tool for outbreak control. It will be years before the NVS and industrial capacity can address anything more than a local outbreak. No new and validated FMD technology, whether for diagnostics, vaccines, or therapeutics, is on the horizon that would rescue the United States in an FMD emergency.
The NBAF is intended, in part, to address this problem. DHS, the state of Kansas, and the city of Manhattan, Kansas are building the NBAF to expand capacity for disease research and MCM R&D for foreign animal and other agricultural diseases. With its large-animal capabilities, NBAF will also assist with the diagnosis and study of additional diseases more rapidly than its predecessor does. NBAF, however, will only reach its full potential if the federal government commits to funding the research its planners envisioned for it.
The fate of another DHS laboratory provides a case in point. The National Biodefense Analysis and Countermeasures Center (NBACC) is a new facility built across two presidential administrations and two parties to meet a national security threat. In the FY 2018 budget request, the Administration proposed elimination of NBACC to fund other priorities. If the federal government approaches the NBAF in similar fashion (a big vision to build, but a small vision to implement long-term programmatic activity once that building is erected), the $1 billion investment could be wasted. If the USDA is the only customer of the lab (much like the FBI has been the only customer of NBACC’s bioforensics lab), this not only eliminates a large opportunity for public-private partnership, but places the lab at the mercy of USDA’s R&D appropriations which are historically a fraction of what is needed.83 It is also the subject of some debate within DHS, USDA, and Congress as to which federal department will assume oversight and funding of NBAF operations. The President’s FY 2018 request would eliminate all agriculture and animal-specific research by the DHS Science and Technology Directorate; this would include agricultural screening and surveillance research and development, as well as foreign animal disease MCM research. The budget request provides no compensatory funding for USDA to take on these missions. As agrodefense is fundamentally a national security concern, it should continue to be a primary responsibility of DHS. While final appropriations language may reject these proposals, they speak to a diminishment of support from the Executive Branch for agriculture and agrodefense research.
In accordance with Recommendation 27 of the Blueprint for Biodefense to prioritize innovation and incrementalism in medical countermeasure development, the Commission proposes the following:
The Secretaries of Agriculture and Homeland Security should establish an antigen bank for foot-and-mouth disease virus. This recommendation is consistent with the Commission’s broader recommendation for federal stakeholders to establish a bank of antigen payloads to operationalize a plug-and-play strategy using proven platform technologies for use in emergencies. The Secretaries should ensure that the acquisition of any such antigen bank is tied to a business plan, to establishment of policies for vaccine usage, and to the National Biodefense Strategy. Further, the Secretary of Homeland Security, in coordination with the Secretary of Agriculture, should develop a business plan for the operation of the National Bio- and Agrodefense Facility, one that would engage the public and private sectors; consider domestic and global markets for agrodefense research and development; and identify a dollar figure that defines the need and the opportunity. In the development of this plan, the Secretary of Homeland Security should issue a Request for Information to assess market opportunity for agricultural research in high-containment laboratories. The Secretary should submit the business plan to congressional committees of jurisdiction, including homeland security and agriculture authorizers and appropriators; future Department of Homeland Security and Department of Agriculture budget requests should align with the plan.
Conclusion
Nearly all federal departments and a few independent agencies contribute directly or indirectly to the protection of American livestock. So do SLTT governments, and so does industry through the efforts of producers, veterinarians, biotechnology companies, and many others. Finding a way to coordinate them is not an easy charge. While a higher priority has understandably been placed to date on protecting human health from intentionally introduced, accidentally released, and naturally occurring infectious diseases, the increasing rate of emerging and reemerging zoonotic disease accompanied by the overt statements and attempts by those with nefarious intent to attack food and agriculture, indicate the necessity to exert more effort to combat threats, eliminate vulnerabilities, and reduce consequences associated with this sector.
The Administration must improve agrodefense efforts at the departmental level and among the interagency. Departmental efforts should be assessed and redirected per the forthcoming National Biodefense Strategy and along the points outlined in this report. One of the most important elements that could materialize from the Strategy is the emergence of departmental ownership of agrodefense. DHS investments in NBAF development, and USDA’s commitment to funding response activities, demonstrate an acknowledgement of the threat. However, current funding levels in areas such as biosurveillance and MCM are insufficient to address mission needs. Furthermore, political leadership and policy coordination, particularly that which acknowledges the intentional dimension of agricultural preparedness, require strengthening. Agrodefense in many ways appears to be an orphan, with long-view funding and policy priority finding a home in neither DHS nor USDA.
Federal investment in the mission space is also temporally lopsided, with more attention and funding brought to bear on the issue when disaster strikes, rather than beforehand. This situation leads inevitably to the incursion of major costs and losses. Such a disparity should be rectified. Budget requests should be submitted and reviewed by OMB and Congress in unified fashion. Beyond the recommendation in this report for such a unified approach to agrodefense budgeting, the Commission will be issuing further analysis of how a more integrated approach can benefit all biodefense efforts. Assessment of capabilities, accountability for these capabilities, and transparency in OMB budget and performance submissions are needed.
The interagency nature of agrodefense means that many congressional committees oversee agrodefense efforts. The House and Senate Committees on Agriculture and Homeland Security should lead these oversight efforts to ensure that all requirements for securing our agricultural enterprise are met. These Committees should both continue and expand previous efforts and increase their direction to the Executive Branch. The Farm Bill provides a significant opportunity every five years to do this legislatively.
In the 115th Congress, Representative David Young and Senator Pat Roberts introduced legislation that would delineate agrodefense-related responsibilities within the Department of Homeland Security. Signed into law in June 2017,84 these bills reflect congressional recognition of the need to establish some degree of ownership of the defense of F&A mission within the Executive Branch. The Commission’s recommendation for further improvements could be directed via the Farm Bill and other authorization and appropriations vehicles.
While many experts agree that bureaucratic silos of the kind that may inhibit collaboration or information sharing do indeed exist, some silos do appear to be thinning over time. Breaking down all bureaucratic stovepipes may never be possible, so the more apt question may be whether it is possible to make the interaction of those silos more efficient and effective, such as through more joint steering committees. While it is important to put in place policies and even statutes that require collaborative effort, the human beings who implement that effort have to want to do so. Examples of success are often based not on policy and law, but on personnel with long-standing relationships across the interagency and the public/private divide, and who want to drive progress.
With each passing year, new threats are discovered that could have severe, long-lasting impacts on animal agriculture. Some of these threats arise at home, and others come from abroad, necessitating concerted effort not just domestically but also internationally. Even with optimized levels of federal leadership, coordination, and funding in place, a common sense of ownership of the challenge, from governmental and non-governmental stakeholders alike, will be necessary. It is essential that our animals, our lives, and our economy are not left vulnerable. The Commission believes that the implementation of the proposals contained in this report is an important step toward that end.
Proposed Congressional Oversight Hearings
 Congressional oversight must ensure that federal departments and agencies meet congressional and other mandates, and in a coordinated fashion. The following proposed hearing topics reflect recommendations discussed in this report, and raise additional ideas for consideration. Parentheticals at the end of each description direct the reader to relevant recommendations in the Blueprint for Biodefense.
Congressional oversight must ensure that federal departments and agencies meet congressional and other mandates, and in a coordinated fashion. The following proposed hearing topics reflect recommendations discussed in this report, and raise additional ideas for consideration. Parentheticals at the end of each description direct the reader to relevant recommendations in the Blueprint for Biodefense.
Animal Disease Reporting
A nationally notifiable animal disease system akin to the existing system for human disease would enhance surveillance and detection of biological threats. A proposed National List of Reportable Animal Diseases has been offered by USDA, but not yet finalized. What is the status of implementation? Will the final rule reflect both the mission need as well as stakeholder input? How could the list be integrated into a system by which states and other owners of disease information could willingly and comfortably report disease incidence? (See Recommendations 7, 14)
House Committees:
- Agriculture
- Homeland Security
- Natural Resources
Senate Committees:
- Agriculture, Nutrition, and Forestry
- Environment and Public Works
- Homeland Security and Governmental Affairs
Biodefense And Agrodefense Strategies
In what ways is agrodefense being addressed and incorporated into the National Biodefense Strategy? Is it receiving the emphasis that the F&A sector requires as a national asset? (See Recommendation 3)
House Committees:
- Agriculture
- Armed Services
- Budget
- Energy and Commerce
- Homeland Security
- Oversight and Government Reform
Senate Committees:
- Agriculture, Nutrition, and Forestry
- Armed Services
- Budget
- Health, Education, Labor, and Pensions
- Homeland Security and Governmental Affairs
Biosurveillance
The United States lacks a comprehensive biosurveillance and detection standard and capability. An integrated biosurveillance function exists in statute, but has been difficult to realize. The program designed to do this, the National Biosurveillance and Integration System, was eliminated in the President’s Budget Request for FY 2018. What would it take to bring agencies with biosurveillance responsibilities, including for animal agriculture and wildlife, together in a trusted, information-sharing environment? What is the needed end-state for a continuous capability to detect, validate, and warn of any biological threat, including agricultural threats, within the United States? Many questions about wildlife zoonoses remain, including the ecology of material threats like Yersinia pestis, and how changing climate patterns will affect the disease distribution of pathogens like avian influenza. How can we achieve a comprehensive and effective national surveillance architecture if we do not invest to answer these scientific questions? (See Recommendations 7, 11, 12, 13, 14)
House Committees:
- Agriculture
- Energy and Commerce
- Homeland Security
- Natural Resources
- Oversight and Government Reform
- Veterans Affairs
Senate Committees:
- Agriculture, Nutrition, and Forestry
- Environment and Public Works
- Energy and Natural Resources
- Health, Education, Labor, and Pensions
- Homeland Security and Governmental Affairs
- Veterans Affairs
Food Supply Protection and Response
The F&A critical infrastructure sector is a distributed and highly complex system. Many efforts have been made to reduce its vulnerability to terrorism and other insults. HSPD-9 (2004) and the DHS F&A Sector Specific Plan (2010), among other policy documents, guide protection of this sector. Have these and other plans been updated, exercised, and sufficiently funded? Are they integrated with related efforts for biosurveillance, attribution, decontamination, and remediation? How will USDA, FDA, CDC, and other federal agencies respond if a terrorist attack impacts the food supply? How can PPP in this area be improved? What efforts and funding are still required to protect the food supply, including plants? Who and in what state is planning for decontamination and remediation to make food processing plants operational again after an incident? (See Recommendations 3, 9, 10)
House Committees:
- Agriculture
- Energy and Commerce
- Homeland Security
- Natural Resources
Senate Committees:
- Agriculture, Nutrition, and Forestry
- Environment and Public Works
- Health, Education, Labor, and Pensions
- Homeland Security and Governmental Affairs
Funding of Preparedness and Response Efforts
Funding for federal agrodefense programs is spread amongst a number of Departments and their corresponding activities. Although HSPD-9 provides a basic framework of agrodefense roles at each phase of preparedness, much of the federal investment in agricultural defense comes in the response phase, leading to greater costs and damages when calamity strikes. The CCC provides significant support to USDA to react to crises, but is not currently utilized in developing more robust preparedness efforts up front. What steps can departments and agencies take to better coordinate their agrodefense spending? What incentives might there be to encourage more investment in preparedness and prevention efforts in advance of a threat to food and agriculture? Is there an opportunity for CCC funds to be used for USDA prevention and mitigation efforts? (See Recommendations 4, 7)
House Committees:
- Agriculture
- Appropriations
- Budget
- Energy and Commerce
- Homeland Security
Senate Committees:
- Agriculture, Nutrition, and Forestry
- Appropriations
- Budget
- Health, Education, Labor, and Pensions
- Homeland Security and Governmental Affairs
Global Health Response
The world lacks a global health response apparatus that can react quickly and insert public health teams to respond to human, animal, and plant outbreaks. What is the current global response capacity and in what ways is it not meeting needs? How can international efforts be evaluated and better coordinated? What is the status of current global health response programs and how can they show more progress? What level of funding would be necessary? What lessons can be learned from recent outbreaks in animals, such as HPAI in China? (See Recommendation 33)
House Committees:
- Agriculture
- Armed Services
- Foreign Affairs
- Energy and Commerce
- Natural Resources
Senate Committees:
- Agriculture, Nutrition, and Forestry
- Armed Services
- Foreign Relations
- Health, Education, Labor, and Pensions
Operational Response And Coordination
In the midst of a crisis, operational leadership is critical to successful outcomes. What is the status of response and recovery planning and recovery efforts for high consequence infectious disease scenarios at all levels of government? What further capabilities do responders, particularly those at the local level, require to combat threats to F&A? What can be done to further multi-agency coordination in this area? How can we increase training efforts related to existing plans and protocols? How can we strengthen relationships and communications among the responsible agencies, to ensure operational leadership? (See Recommendations 16, 17)
House Committees:
Agriculture
Homeland Security
Senate Committees:
Agriculture, Nutrition, and Forestry
Homeland Security and Governmental Affairs
Workforce
The national veterinary workforce trained to prevent, detect, and respond to livestock outbreaks of foreign animal diseases is limited. Yet it is this profession that is responsible for protecting animal health and welfare and, therefore, all of the elements of this sector important to human health and the economy. The National Veterinary Emergency Response Teams (NVERT) are the core federal response capacity needed for large animal health situations. Are the available NVERTs sufficient to respond to an animal emergency of catastrophic proportions? Is a USAJOBS-based application requirement the best way to invite and incentivize private sector veterinary professionals into the system? Is the Public Service Loan Forgiveness Program a potential vehicle for expanding the workforce? How can the barriers of entry for interested veterinarians be lowered?
House Committees:
Agriculture
Appropriations
Senate Committees:
Agriculture, Nutrition, and Forestry
Appropriations
Appendix A: Methodology
Established in 2014, the Bipartisan Commission on Biodefense (formerly the Blue Ribbon Study Panel on Biodefense) informs U.S. biodefense efforts and provides recommendations for needed change. The Commission, supported by seven ex officio members and funds from foundations, industry, and individual donors, assesses where the United States falls short in addressing biological terrorism, warfare, accidents, and emerging, reemerging, and other naturally occurring infectious diseases. Information-gathering is achieved primarily through public and private meetings and literature research, and recommendations are issued in the form of reports. The Commission works to educate all stakeholders and the public about its findings through these reports, public appearances, and other communications platforms.
Research Questions
In order to assess gaps in the animal agrodefense enterprise, the Commission developed the following research questions:
1.Are our priorities correct?
2.Are our investments commensurate with the challenge?
3.Can we benefit by rebalancing investments, or is new funding required?
4.What have we done that has brought a significant return on investment?
5.What else should we be doing that we are not?
Research Activity
For this special focus report, the Commission reviewed scientific studies; reports by congressional and presidential commissions; presidential directives; statute and proposed legislation; GAO reports; and federal strategies, plans, budgets, organizational constructs, and programs related to defense against deliberately introduced, accidentally released, and naturally occurring biological events with catastrophic potential. This review: 1) informed the Commission’s assessment of the comprehensiveness of efforts to address postulated and actual agrodefense challenges; 2) informed the Commission’s determination of how the understanding of the threat, knowledge base, and elements of the agrodefense enterprise should change in light of this assessment; and 3) shaped the structure and topics of the agrodefense special focus meeting held by the Commission on January 26, 2017 in Manhattan, Kansas.
Agrodefense Special Focus Meeting
The Commission organized this special focus meeting around the major activities that comprise the biodefense enterprise at large: prevention, deterrence, preparedness, surveillance and detection, response, recovery, attribution, and mitigation. Two Commissioners, former Senate Majority Leader Tom Daschle and former Homeland Security Advisor Ken Wainstein, co-chaired the meeting and received: 1) information regarding national agrodefense policy, departmental and agency programmatic activities, and legislative matters; and 2) statements from a sitting member of Congress, former federal officials, current state officials, academic and private sector representatives, thought leaders, and subject matter experts. After the meeting, Commission staff summarized major insights, areas for improvement, and recommendations articulated by meeting speakers, and conducted preliminary high-level analysis of the meeting. See Appendix C for the meeting agenda and speakers.
Analysis
Commission staff qualitatively analyzed the information gleaned from their research and from the special focus meeting. Staff evaluated facts, findings, and recommendations provided by meeting speakers and through other means, including policy research and interviews with subject matter experts and former high-level officials. Throughout the process, the five research questions above provided the basis for assessment. This approach allowed Commissioners and staff to identify continuing organizational, legal, policy, and programmatic issues, and to recommend solutions. Commission staff did not use statistical and other quantitative methods for this study. The study is not considered pseudo-qualitative/quasi-quantitative.
Study Limitations
Funding and other resource constraints prevented the Commission from performing site visits beyond visiting the Biosecurity Research Institute at Kansas State University. The Commission did not assess challenges in protecting the food supply or the plant sector, as these are extensive enterprises in and of themselves and require their own special focus. In addition, some agrodefense programs and policies; intelligence, raw data, and documents; appropriations and budget documents; and other sensitive pieces of information are classified or otherwise unavailable, and were not reviewed by the Commission as this was a wholly unclassified endeavor.
Appendix B: Meeting Agenda and Speakers
The following is the agenda for the special focus meeting at Kansas State University, Manhattan, Kansas. Names and affiliations appear here as they did at the time of the meeting.
Agrodefense: Challenges And Solutions
JANUARY 26, 2017
Opening Remarks
- Former Senate Majority Leader Thomas A. Daschle, Commissioner, Bipartisan Commission on Biodefense
- Former Homeland Security Advisor, Kenneth L. Wainstein, Commissioner, Bipartisan Commission on Biodefense
- President Richard B. Myers, Kansas State University (General, USAF – retired)
Congressional Perspective
- The Honorable Roger Marshall, MD, United States Representative, Kansas
Panel One – Prevention and Deterrence
Challenges and opportunities in reducing risk from agricultural threats. Understanding the challenges of laboratory research in the context of threats to F&A, regulatory regimes, and new technologies. Ways in which outbreaks have demonstrated strengths and weaknesses, with respect to medical countermeasures.
- Stephen Higgs, PhD, Associate Vice President for Research and Director, Biosecurity Research Institute
- Amy Kircher, DrPH, Director, Food Protection and Defense Institute, University of Minnesota
- Steve Parker, MBA, MSCM, Head, North America Veterinary Public Health, Merial
Lunch Keynote – Leadership in Protecting the Agricultural Sector
- Bret D. Marsh, DVM, Indiana State Veterinarian
Panel Two – Surveillance and Detection
Key elements of effective agricultural biosurveillance and detection, and continued challenges in the effectiveness of ongoing efforts. Technological and policy challenges for early and reliable detection of environmentally dispersed biological agents to attack agriculture. Key elements of effective animal and plant surveillance and detection architecture, and impediments and opportunities to increase situational awareness for early and accurate disease detection and clinical diagnoses. Requirements for medical countermeasures, including the need for extremely rapid development, distribution, and dispensing.
- Tammy R. Beckham, DVM, PhD, Dean, College of Veterinary Medicine, Kansas State University
- Ali S. Khan, MD, MPH, Dean, College of Public Health, University of Nebraska Medical Center
- Kelly F. Lechtenberg, DVM, PhD, President, Midwest Veterinary Services/Central States Research Center/Veterinary and Biomedical Research Center
Panel Three – Preparedness, Response, Recovery, and Mitigation
Pre- and post-event planning, including the challenges faced by the food, agriculture, and public health communities, and the roles of state, local, and federal governments. Challenges of epidemiology and other tools for characterizing the spread of animal, plant, and foodborne diseases in the United States. Recovery and mitigation, including the challenges posed by cutting edge technology, lack of agreement regarding state and federal responsibilities, and implications for future preparedness.
- Jackie McClaskey, PhD, Secretary, Kansas Department of Agriculture
- D. Charles Hunt, MPH, State Epidemiologist and Director, Bureau of Epidemiology and Public Health Informatics, Kansas Department of Health and Environment
- C. J. Mann, DVM, Chief Executive, Empryse Group
Closing Remarks
- President Richard B. Myers, Kansas State University (General, USAF – retired)
- Former Homeland Security Advisor, Kenneth L. Wainstein, Commissioner, Bipartisan Commission on Biodefense
- Former Senate Majority Leader Thomas A. Daschle, Commissioner, Bipartisan Commission on Biodefense
Appendix C: Acronyms
References
- Food and Drug Administration, U.S. Department of Agriculture, and Department of Homeland Security. (2015). Food and Agriculture Sector Specific Plan.
- Economic Research Service. (2016). What is agriculture’s share of the overall U.S. economy? Washington, DC: U.S. Department of Agriculture. Retrieved from https://www.ers.usda.gov/data-products/chart-gallery/gallery/chart-detail/?chartId=58270.
- Breeze, R. (2004) Agroterrorism: Betting Far More than the Farm. Biosecurity and Bioterrorism: Biodefense Strategy, Practice and Science4:251-264.
- Monke, J. (2007, March 12). Agroterrorism: Threats and Preparedness. Congressional Research Service, Report RL32521.
- Monke, J. (2007, March 12). Agroterrorism: Threats and Preparedness. Congressional Research Service, Report RL32521.
- Olsen, D. (2012). Agroterrorism: Threats to America’s Economy and Food Supply. Washington, DC: Federal Bureau of Investigation. Retrieved from https://leb.fbi.gov/2012/february/agroterrorism-threats-to-americas-economy-and-food-supply.
- Institute of Medicine. (2012). Improving Food Safety through a One Health Approach – Workshop Summary. Washington, DC: The National Academies Press. doi:org/10.17226/13423.
- Radosavljevic, V., Finke, E. J., & Belojevic, G. (2016). Analysis of Escherichia Coli O104:H4 Outbreak in Germany in 2011 Using Differentiation Method for Unusual Epidemiological Events. Cent Eur J Public Health, 24(1), 9-15. doi:10.21101/cejph. a4255.
- Beef Checkoff Program. (2017). Fact Sheet: Industry Economics: The Economic Effect of Foot-and-Mouth Disease. Centennial, CO: National Cattlemen’s Beef Association. Retrieved from https://www.footandmouthdiseaseinfo.org/factsheetindustryeconomics.aspx.
- Atkinson, N. (1999). The Impact of BSE on the UK Economy. Economics (International) Division, Ministry of Agriculture, Fisheries and Food, London: England. Retrieved from www.veterinaria.org/revistas/vetenfinf/bse/14Atkinson.html.
- Amelinckx, A. (December 2013). The Top 10 Farm Crimes in 2013. Modern Farmer.
- Government Accountability Office. (April 2017). Avian Influenza: USDA has taken actions to reduce risks but needs a plan to evaluate its efforts (GAO-17-360). Washington, DC: Government Accountability Office.
- Lee, D.H., Bahl, J., Torchetti, MK, et al. (July 2016). Highly Pathogenic Avian Influenza Viruses and Generation of Novel Reassortants, United States, 2014-2015. Emerging Infectious Diseases 22(7):1283-1285.
- Johnson K.K., Seeger, R.M., and Marsh, T.L. (2nd Quarter 2016). Local Economies and Highly Pathogenic Avian Influenza. Choices 31(2).
- Government Accountability Office. (April 2017). Avian Influenza: USDA has taken actions to reduce risks but needs a plan to evaluate its efforts (GAO-17-360). Washington, DC: Government Accountability Office.
- Newton, J. and Kuethe, T. (2015, June 5). Economic Implications of the 2014-2015 Bird Flu. Farmdoc Daily (5):104. Department of Agricultural and Consumer Economics, University of Illinois at Urbana-Champaign.
- Elam, T.E. (2015, June 29). Economic Losses from the 2015 Highly Pathogenic Avian Flu Outbreak. FarmEcon LLC.
- Shane, S. (September 2015). Lessons learned from the recent US HPAI epidemic. Poultry World, Retrieved from https://www.poultryworld.net/Health/Articles/2015/9/Lessons-learned-from-the-recent-US-HPAI-epornitic-2693194W/.
- Johnson, R. (January 2010). Potential Farm Sector Effects of 2009 H1N1 “Swine Flu”: Questions and Answers (R40575). Washington, DC: Congressional Research Service.
- Witsanu, A., McCarl, B.A., and Bessler, D. (Summer 2011). The Effect of H1N1 (Swine Flu) Media Coverage on Agricultural Commodity Markets. Applied Economic Perspectives and Policy 33(2): 241-259.
- Animal and Plant Health Inspection Service. (June 2014). Novel Swine Enteric Coronavirus Diseases (SECD), Case Definition. Washington, DC: U.S. Department of Agriculture.
- Paarlberg, P. L. (April 2014). Updated estimated economic welfare impacts of porcine epidemic diarrhea virus (PEDV) – Working Paper #14-4. W. Lafayette, IN: Purdue University.
- United States Department of Agriculture, Animal and Plant Health Inspection Service, “Swine Enteric Coronavirus Introduction to the United States: Root Cause Investigation Report” (September 2015).
- Taylor, L. H., Latham, S. M., & Woolhouse, M. E. (2001). Risk factors for human disease emergence. Philosophical Transactions of the Royal Society B: Biollogical Sciences, 356(1411), 983-989. doi:10.1098/rstb.2001.0888.
- World Health Organization. (March 2017). Human infection with avian influenza A(H7N9) virus – China. Geneva, Switzerland: World Health Organization. Retrieved from https://www.who.int/csr/don/16-march-2017-ah7n9-china/en/.
- Brahmbhatt, M. and Dutta, A. (January 2008). On SARS Type Economic Effects during Infectious Disease Outbreaks (Policy Research Working Paper 4466). Washington, DC: The World Bank. doi/org/10.1596/1813-9450-4466.
- Lee, J.W. and McKibbin, W.J. (2004). Estimating the global economic costs of SARS. In: Institute of Medicine (US) Forum on Microbial Threats; Knobler, S., Mahmoud, A., and Lemon S, et. al. (Eds). Learning from SARS: Preparing for the Next Disease Outbreak: Workshop Summary. Washington, DC: National Academies Press.
- Centers for Disease Control and Prevention. (December 2003). Revised U.S. Surveillance Case Definition for Severe Acute Respiratory Syndrome (SARS) and Update on SARS Cases — United States and Worldwide. MMWR 52(49): 1202-1206.
- Khan, A. S. (2017, January 26). Panel Two: Surveillance and Detection. Remarks presented at Agrodefense: Challenges and Solutions, a public meeting of the Blue Ribbon Study Panel on Biodefense, in Manhattan, Kansas.
- Breeze, R. (2004). Agroterrorism: Betting More than the Farm. Biosecurity and Bioterrorism: Biodefense Strategy, Practice, and Science 2(4): 1-14.
- Government Accountability Office. (April 2017). Avian Influenza: USDA has taken actions to reduce risks but needs a plan to evaluate its efforts (GAO-17-360). Washington, DC: Government Accountability Office.
- Government Accountability Office. (January 2016). Emerging Animal Diseases: Actions Needed to Better Position USDA to Address Future Risks (GAO-16-132). Washington, DC: Government Accountability Office.
- The White House. (February 2013). Presidential Policy Directive 21: Critical Infrastructure Security and Resilience. Washington, DC: The White House.
- Department of Homeland Security. (January 2008). National Response Plan: Food and Agriculture Incident Annex. Washington, DC: Federal Emergency Management Agency.
- Marsh, B.D. (2017, January 26). Leadership in Protecting the Agricultural Sector. Remarks presented at Agrodefense: Challenges and Solutions, a public meeting of the Blue Ribbon Study Panel on Biodefense, in Manhattan, Kansas.
- Food and Drug Administration, U.S. Department of Agriculture, and Department of Homeland Security. (2015). Food and Agriculture Sector Specific Plan.
- Advisory Panel to Assess Domestic Response Capabilities for Terrorism Involving Weapons of Mass Destruction. (December 1999). First Annual Report to the President and Congress: I. Assessing the Threat, pp. 12-15.
- The White House. (January 2004). Homeland Security Presidential Directive / HSPD-9. Defense of United States Agriculture and Food. Washington, DC: The White House.
- The White House. (April 2004). Biodefense for the 21st Century. Washington, DC: The White House. Retrieved from https://www.virtualbiosecuritycenter.org/library/biodefense-for-the-21st-century.
- Monke, J. (March 2007). Agroterrorism: Threats and Preparedness (RL 32521). Washington, DC: Congressional Research Service.
- House Committee on Agriculture. Examination of Federal and State Response to Avian Influenza. Hearing before the Subcommittee on Livestock and Foreign Agriculture. 114th Congress. July 30, 2015; Senate Committee on Agriculture, Nutrition, and Forestry. Hearing on Highly Pathogenic Avian Influenza: The Impact on the U.S. Poultry Sector and Protecting U.S. Poultry Flocks. Hearing before the full committee. 114th Congress. July 7, 2015.
- House Committee on Agriculture. The Next Farm Bill: Livestock Producer Perspectives. Hearing before the Subcommittee on Livestock and Foreign Agriculture.115th Congress. March 21, 2017; Senate Committee on Agriculture, Nutrition, and Forestry. Hearing from the Heartland: Perspectives on the 2018 Farm Bill from Kansas. Field Hearing. 115th Congress. February 23, 2017. Testimony of Jackie McClaskey.
- House Committee on Agriculture. Foot and Mouth Disease: Are We Prepared? Hearing before the Subcommittee on Livestock and Foreign Agriculture. 114th Congress. February 11, 2016.
- House Committee on Homeland Security. Food for Thought: Efforts to Defend the Nation’s Agriculture and Food. Hearing before the Subcommittee on Emergency Preparedness, Response, and Communications. 114th Congress. February 26, 2016.
- The White House. (January 2004). Homeland Security Presidential Directive / HSPD-9. Defense of United States Agriculture and Food. Washington, DC: The White House.
- Office of Management and Budget. (2017) Analytical Perspectives: Budget of the U.S. Government, Fiscal Year 2017. Washington, DC: U.S. Government Printing Office.
- The White House. (January 2004). Homeland Security Presidential Directive / HSPD-9. Defense of United States Agriculture and Food. Washington, DC: The White House.
- U.S. Food and Drug Administration, Federal Bureau of Investigation, and U.S. Department of Agriculture. (July 2008). Criminal Investigation Handbook for Agroterrorism, p. 34. Washington, DC: Federal Bureau of Investigation.
- U.S. Department of Homeland Security. (October 2016). Nuclear/Radiological Incident Annex to the Response and Recovery Federal Interagency Operational Plans.
- Government Accountability Office. (April 2017). Avian Influenza: USDA has taken actions to reduce risks but needs a plan to evaluate its efforts (GAO-17-360). Washington, DC: Government Accountability Office.
- Shane, S. (September 2015). Lessons learned from the recent US HPAI epidemic. Poultry World, Retrieved from https://www.poultryworld.net/Health/Articles/2015/9/Lessons-learned-from-the-recent-US-HPAI-epornitic-2693194W/.
- Johnson R. (January 2010). Potential Farm Sector Effects of 2009 H1N1 Swine Flu: Questions and Answers. Washington, DC: Congressional Research Service.
- Elam, T.E. (June 2015). Economic Losses from the 2015 Highly Pathogenic Avian Flu Outbreak. Washington, DC: Senate Committee on Agriculture, Nutrition, and Forestry.
- Government Accountability Office. (April 2017). Avian Influenza: USDA has taken actions to reduce risks but needs a plan to evaluate its efforts (GAO-17-360). Washington, DC: Government Accountability Office.
- Johansson, R.C., Preston, W.P., Seltzinger, A.H. (2nd Quarter 2016). Government Spending to Control Highly Pathogenic Avian Influenza,” Choices, 31(2): 1-7.
- Decision Innovation Solutions. (August 2015). Economic impact of highly pathogenic avian influenza (HPAI) on poultry in Iowa. West Des Moines, IA: Iowa Farm Bureau.
- Shane, S. (September 2015). Lessons learned from the recent US HPAI epidemic. Poultry World, Retrieved from https://www.poultryworld.net/Health/Articles/2015/9/Lessons-learned-from-the-recent-US-HPAI-epornitic-2693194W/.
- Johansson, R.C., Preston, W.P., Seltzinger, A.H. (2nd Quarter 2016). Government Spending to Control Highly Pathogenic Avian Influenza,” Choices, 31(2): 1-7.
- United States Government Accountability Office. (December 2015.) Emerging Animal Diseases: Actions needed to better position USDA to address future risks (GAO-16-132). Washington, DC: Government Accountability Office.
- Government Accountability Office. (April 2017). Avian Influenza: USDA has taken actions to reduce risks but needs a plan to evaluate its efforts (GAO-17-360). Washington, DC: Government Accountability Office.
- Government Accountability Office. (April 2017). Avian Influenza: USDA has taken actions to reduce risks but needs a plan to evaluate its efforts (GAO-17-360). Washington, DC: Government Accountability Office.
- Government Accountability Office. (April 2017). Avian Influenza: USDA has taken actions to reduce risks but needs a plan to evaluate its efforts (GAO-17-360). Washington, DC: Government Accountability Office.
- The White House. (April 2004). Biodefense for the 21st Century. Washington, DC: The White House. Retrieved from https://www.virtualbiosecuritycenter.org/library/biodefense-for-the-21st-century.
- United States Department of Agriculture. Feral swine disease surveillance. Retrieved from https://www.aphis.usda.gov/aphis/ourfocus/wildlifedamage/programs/nwrc/sa_nwdp/ct_feral_swine
- National Wildlife Disease Program. (February 2014). Feral swine as biosentinels for Bacillus anthracis: Program Activity Report. Washington, DC: United States Department of Agriculture.
- Marsh, B.D. (2017, January 26). Leadership in Protecting the Agricultural Sector. Remarks presented at Agrodefense: Challenges and Solutions, a public meeting of the Blue Ribbon Study Panel on Biodefense, in Manhattan, Kansas.
- Bevins, S. N., Pedersen, K., Lutman, M. W., Baroch, J. A., Schmit, B. S., Kohler, D., . . . DeLiberto, T. J. (2014). Large-scale avian influenza surveillance in wild birds throughout the United States. PLoS One, 9(8), e104360. doi:10.1371/journal.pone.0104360.
- U.S. Department of Agriculture. (2006). An Early Detection System for Highly Pathogenic H5N1 Avian Influenza in Wild Migratory Birds: U.S. Interagency Strategic Plan. Washington, DC: U.S. Department of Agriculture.
- U.S. Department of Agriculture. (June 2015). Surveillance plan for highly pathogenic avian influenza in waterfowl in the United States. Washington, DC: U.S. Department of Agriculture.
- Government Accountability Office. (April 2017). Avian Influenza: USDA has taken actions to reduce risks but needs a plan to evaluate its efforts (GAO-17-360). Washington, DC: Government Accountability Office.
- Congress asserted the need for such integration in the statutory provision that established the NBIC. See P.L. 110-53, Implementing Recommendations of the 9/11 Commission Act of 2007 (6 USC 195b).
- Homeland Security Council. (2004, January 30). Defense of United States Agriculture and Food. Washington, DC: The White House.
- U.S. Department of Agriculture. (2015). Early Detection and Monitoring for Avian Influenzas of Significance in Wild Birds: A U.S. Interagency Strategic Plan. Washington, DC: U.S. Department of Agriculture; U.S. Department of Agriculture. (2015). 2015 Surveillance Plan for Highly Pathogenic Avian Influenza in Waterfowl in the United States.
- Food, Conservation and Energy Act, Public Law 110-246 (2008).
- United States Department of Agriculture. (September 2016). U.S. National List of Reportable Animal Diseases (NLRAD) Framework.
- United States Government Accountability Office. (December 2015). Emerging Animal Diseases: Actions needed to better position USDA to address future risks. GAO-16-132.
- U.S. Department of Agriculture, Animal and Plant Health Inspection Service. “Proposal for a U.S. National List of Reportable Animal Diseases (NLRAD) Concept Paper”, (July 2014).
- The White House. (January 2004). Homeland Security Presidential Directive/HSPD-9. Defense of United States Agriculture and Food. Washington, DC: The White House.
- Hsu, S., Chen, T., & Wang, C. (2010). Efficacy of Avian Influenza Vaccine in Poultry: A Meta-analysis. Avian Diseases, 54(4), 1197-1209.
- U.S. Department of Agriculture. (January 11, 2016). 2016 HPAI Preparedness and Response Plan.
- U.S. Department of Agriculture. (September 2014). Foot-and-Mouth disease vaccination policy in the United States. Washington, DC: U.S. Department of Agriculture.
- U.S. Department of Agriculture. (September 2014). Foot-and-Mouth disease vaccination policy in the United States. Washington, DC: U.S. Department of Agriculture.
- Knight-Jones, T., Robinson, L., Charleston, B., Rodriguez, L.L., Gay, C.G., Sumption, K., Vosloo, W. 2016. Global foot-and-mouth disease research update and gap analysis: 1 – Overview of global status and research needs. Transboundary and Emerging Diseases. 63:3-13. doi:10.1111/tbed.12528.
- Securing our Agriculture and Food Act, Public Law 115-43. (2017).
Copyright © 2017 by the Bipartisan Commission on Biodefense at the Potomac Institute for Policy Studies. All rights reserved.
Cover and graphics by Delbridge Design.
Base cover image courtesy of Getty Images/feoris.
Bipartisan Commission on Biodefense. Special Focus: Defense of Animal Agriculture. Bipartisan Commission on Biodefense: Washington, DC. October 2017.

